Abstract
Aims
The aim of this study was to determine clinical variables associated with posaconazole exposure among adult patients with haematological malignancies who received posaconazole tablets for prophylaxis of invasive fungal infections (IFIs).
Methods
The study population included adult patients with haematological malignancies who received posaconazole delayed‐release tablets for prophylaxis of IFIs after induction chemotherapy for acute leukaemia or graft‐versus‐host‐disease (GVHD) complicating hematopoietic stem cell transplantation (HSCT) in the period January 2016–December 2017.
Results
Sixty‐six consecutive patients with 176 posaconazole C min were included for evaluation in the study. Subtherapeutic posaconazole concentrations (< 0.7 mg l−1) were observed at least once in 33.3% of patients (22/66), and overall in 17.0% of therapeutic drug monitoring (TDM) episodes (30/176). At multilevel linear regression, use of PPIs (P = 0.008), use of intermediate or high dose steroids (>0.7 mg kg−1 daily) (P = 0.022) and male gender (P = 0.025) were significantly associated with decreased C min, whereas time from starting therapy (P = 0.032) was associated with increased C min in our patient population.
Conclusion
Posaconazole exposure during treatment with delayed‐released tablet formulation may be affected by the use of PPIs and/or of intermediate or high dose steroids.
Keywords: clinical pharmacology, drug interactions, infectious diseases, pharmacokinetics, therapeutic drug monitoring, transplantation
What is Already Known about this Subject
High pharmacokinetic variability of posaconazole oral suspension may often lead to suboptimal concentrations of posaconazole.
Delayed‐release tablet formulation was developed with the intention of overcoming the limitations of oral posaconazole suspension.
Variability in posaconazole exposure is still observed even with the delayed‐released tablet.
What this Study Adds
Use of proton pump inhibitors (PPIs) was associated with a 45% reduction in C min.
Use of steroids (≥0.7 mg kg−1 daily) was associated with a 44% reduction in C min.
TDM of posaconazole tablet could be especially important for patients receiving both PPIs and steroids.
Introduction
Invasive fungal infections (IFIs) represent a major cause of morbidity and mortality among patients with acute leukaemia who receive induction chemotherapy and among haematopoietic stem cell transplant (HSCT) recipients with graft‐versus‐host disease (GVHD) 1. A retrospective study carried out among HSCT recipients in the period 2007–2012 showed that the overall incidence of IFIs was 11.3%, and that the majority of these were due to Aspergillus spp. and/or to other moulds 2.
Current guidelines recommend the use of posaconazole as primary prophylaxis for the prevention of IFIs in acute myeloid leukaemia (AML) and for patients undergoing HSCT with GVHD 3. Posaconazole is a second‐generation triazole agent, which is currently available in two oral formulations: a suspension and delayed‐release tablets. The oral suspension has saturable absorption, that is improved by food or a high fat meal, and whose oral bioavailability may be decreased by the co‐administration of gastric acid‐lowering agents 4, 5. A highly variable pharmacokinetic profile of posaconazole oral suspension often leads to suboptimal concentrations of posaconazole with an attendant risk of breakthrough IFIs 6.
A delayed‐release tablet formulation was developed with the intention of overcoming some of the limitations of oral posaconazole suspension. Although the new formulation has a generally improved oral bioavailability, there are conflicting results in terms of posaconazole serum trough concentrations (C min) observed in real‐life following administration of the standard 300 mg once‐daily dose. Some authors have documented better drug exposure 7 that is independent of the regimen, clinical status and/or co‐medications 8, 9, whereas others showed that attainment of therapeutic concentrations may still be problematic 10, 11. The variability of posaconazole exposure may be caused by erratic absorption as well as enhanced clearance (CL). Furthermore, posaconazole is very highly bound to plasma proteins and its elimination is mediated by the UDP‐glucuronosyltransferase (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=988) 1A4 12. Accordingly, hypoalbuminaemia and/or the presence of factors that may upregulate the activity of UGT1A4 may lead to an increase of posaconazole CL. A recent case report suggested that in a patient with AML receiving posaconazole as delayed‐release tablets, hypoalbuminaemia and hyperbilirubinaemia may have been responsible for an enhancement of posaconazole CL, leading to subtherapeutic concentrations 13.
The aim of this single‐centre retrospective study was to determine risk factors associated with subtherapeutic concentrations of posaconazole among adult patients with haematological malignancies who received posaconazole tablets for prophylaxis of IFIs.
Methods
Study design
This was a single‐centre retrospective study that included adult patients with haematological malignancies who were admitted at the Clinic of Haematology, Santa Maria della Misericordia University Hospital of Udine, Italy in the period January 2016–December 2017. All patients received posaconazole delayed‐release tablets for prophylaxis of IFIs after induction chemotherapy for acute leukaemia or GVHD complicating HSCT. Therapeutic drug monitoring (TDM) of posaconazole was performed on all patients. The study was approved by the Regional Ethics Committee. Written informed consent was waived due to the retrospective and observational nature of the investigation.
All the patients were recommended to take posaconazole tablets with food. Therapy was started with an initial loading dose of 300 mg every 12 h on Day 1 and continued in the subsequent days with a maintenance dose of 300 mg once daily until the first episode of TDM, which was performed at steady‐state (after 4 or more days from treatment initiation). The target C min was >0.7 mg l−1 according to current recommendations for prophylaxis 14, 15. In the presence of subtherapeutic C min (≤ 0.7 mg l−1), the maintenance dose was increased to 400 mg. Blood samples for determination of trough levels (C min) were collected in the morning immediately prior to the scheduled intake of posaconazole tablets. Compliance was verified at each TDM instance and reported in the patient data sheet. Samples were immediately delivered to the Institute of Clinical Pharmacology, where they were centrifuged to obtain serum. The separated serum samples were stored at −20°C until analysed (once weekly on Tuesdays). Posaconazole concentrations were estimated using a previously described liquid chromatography–tandem mass spectrometry analytic method 16. The intra‐ and inter‐day coefficients of variation were 0.17–1.02% and 0.86–1.67%, respectively. The lower limit of quantitation was 0.04 mg l−1 and the limit of detection was 0.02 mg l−1.
Data on demographic and clinical characteristics (age, weight, height, gender, underlying haematological disease, presence of acute or chronic GVHD, duration of posaconazole treatment), laboratory parameters (alanine aminotransferase, serum albumin, serum creatinine, serum bilirubin concentrations) and on all co‐medications (with dosages) were retrieved for each patient at baseline and at each TDM episode. The following risk factors potentially affecting posaconazole absorption were collected for evaluation: use of proton pump inhibitors (PPIs), presence of mucositis and of diarrhoea 11. Additionally, the following risk factors potentially affecting the CL of posaconazole were collected: presence of hypoalbuminaemia, presence of hyperbilirubinaemia 13 and use of intermediate or high dose steroids (defined as >0.7 mg kg−1 daily).
Statistical analysis
The Kolmogorov–Smirnov test was used to assess whether clinical data were normally distributed. The mean ± standard deviation, median and 25th–75th interquartile ranges (IQR) were used for descriptive statistics. Intra‐individual and inter‐individual variability of posaconazole C min was assessed by calculating the median of the coefficient of variation (CV) of all the C min measured in a single patient and the CV of the average C min of each patient, respectively. Univariate and multivariate linear mixed‐effect models (with a random effect for patient accounting for correlation amongst repeated measurements of the same subjects) were performed to identify the independent predictors of posaconazole C min. Multivariate stepwise backward analysis included all variables significant at P ≤ 0.200 in the univariate analysis. A Kruskal–Wallis test was used to compare continuous data among groups. Bonferroni correction for multiple comparisons was applied, as appropriate. A P‐value <0.05 was required for statistical significance. All statistical analysis and plotting were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 17, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 18, 19.
Results
Sixty‐six consecutive patients were included in the study. Patient demographics and clinical characteristics are summarized in Table 1. Males accounted for 65.2% (43/66) of the population. Median (IQR) age and body weight of the cohort were 58 years (49–64 years) and 75 kg (62–88.8 kg), respectively. The most frequent underlying disease was AML (46/66; 69.7%) and 28 patients (28/66, 42.4%) underwent HSCT. Most of the patients received posaconazole prophylaxis after induction chemotherapy (48/66; 72.7%). Fifty‐one patients (51/66, 77.3%) were co‐treated with PPIs (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7260, n = 48, median [IQR] dose of 40 [20–40] mg daily; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4279, n = 2, median [IQR] doses of 60 [50–60] mg daily; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7290, n = 1, dose of 40 mg daily). Eighteen patients (18/66, 27.3%) received co‐treatment with intermediate or high dose steroids (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7088, n = 14 patients, median [IQR] doses of 90 [80–140] mg daily; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7096, n = 4 patients, median [IQR] doses of 75 [52.5–95] mg daily).
Table 1.
Patient demographics and posaconazole treatment
| Total number of patients | 66 |
| Age (years) | 58 (49–64) |
| Gender (M/F) | 43/23 |
| Body weight (kg) | 75.0 (62.0–88.8) |
| Albumin (g l −1 ) | 34.7 (31.4–37.0) |
| Total bilirubin (mg dl −1 ) | 0.84 (0.58–1.37) |
| Alanine‐aminotransferase (IU l −1 ) | 38.0 (24.0–64.0) |
| Underlying disease | |
| AML | 46 (69.7) |
| ALL | 5 (7.6) |
| Lymphoma/Chronic lymphoproliferative diseases | 8 (12.1) |
| MM | 3 (4.5) |
| MDS/Chronic myeloproliferative diseases | 4 (6.1) |
|
Number of HSCT
Indications for posaconazole |
28 |
| Prophylaxis after induction chemotherapy | 48 (72.7) |
| Prophylaxis for GVHD | 18 (27.3) |
| Posaconazole treatment | |
| Dose (mg)° | 300 (200–400) |
| Total number of C min | 176 |
| C min (mg l −1 ) | 1.17 (0.86–1.59) |
| Number of TDM instances | 2 (1–3) |
| Time (days) to first TDM assessment | 6 (4–10) |
| C min (mg l −1 ) at first TDM assessment | 0.93 (0.68–1.33) |
| Clinical outcome | |
| Successful prophylaxis | 61 (92.5) |
| Dead for other reasons | 3 (4.5) |
| Fungal infection (possible) | 2 (3.0) |
ALL, acute lymphocytic leukaemia; AML, acute myeloid leukaemia; GVHD, graft‐versus‐host disease; MDS, myelodysplastic syndrome; MM, multiple myeloma; TDM, therapeutic drug monitoring. Data for continuous variables are presented as median (IQR) or as median (min‐max)°, and data for dichotomous variables are presented as number (%).
Overall, 176 posaconazole C min were included for evaluation. Median (IQR) posaconazole C min was 1.17 mg l−1 (0.86–1.59 mg l−1), but concentrations after standard 300 mg dose ranged between 0.17 and 4.53 mg l−1. The overall CV% of posaconazole C min was 50.9%. Inter‐individual and intra‐individual variability were 42.7% and 32.6%, respectively. Low posaconazole concentrations (C min < 0.7 mg l−1) were observed in 33.3% of patients (22/66), with an overall frequency of 17.0% of TDM episodes (30/176). Dosage escalation up to 400 mg daily was required in 9/66 patients (13.6%). Among HSCT patients, median [IQR] posaconazole C min during co‐administration of intermediate or high dose steroids was significantly lower than after de‐escalation of steroid dosage (0.90 [0.64–1.42] mg l−1 vs. 1.37 [1.0–1.79] mg l−1; P = 0.008). Breakthrough possible aspergillosis were documented in two patients. The posaconazole C min at Day 5 was 1.52 and 1.74 mg l−1.
Figure 1 summarizes distributions of posaconazole C min in relation to the duration of treatment among the studied patients. Overall, a statistically significant progressive increase of posaconazole C min was observed in relation to the duration of treatment (P = 0.005). After correction for multiple comparisons, significant differences in median posaconazole C min were observed between week 1 and 4 (C min of 0.9 vs. 1.27 mg l−1, P = 0.007) and week 1 and >4 (C min of 0.9 vs. 1.37 mg l−1, P < 0.001).
Figure 1.
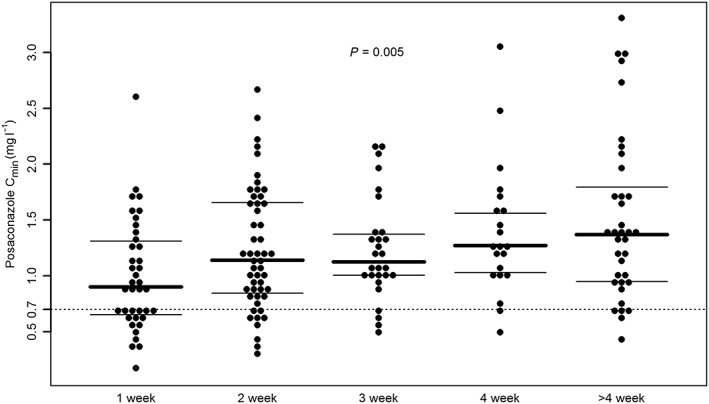
Beeswarm plot of the distribution of posaconazole trough concentrations (n = 176) following administration of posaconazole tablets in hematologic patients, in relation to week of treatment. Solid lines identify median and 25th–75th percentiles within each group. The dashed line identifies posaconazole target level for prophylaxis (C min > 0.7 mg l−1) of fungal infections. A P‐value = 0.005 was obtained at Kruskal–Wallis test. A significant difference after post‐hoc Bonferroni correction was observed between week 1 and week 4 (P = 0.007) and week 1 and week >4 (P < 0.001)
Univariate and multivariate mixed‐effect linear regression analysis of the clinical variables associated with posaconazole C min are reported in Table 2. Multivariate analysis showed that in patients receiving posaconazole tablets, male gender, co‐treatment with PPIs or with steroids was associated with a 28%, 45% or 44% reduction in C min. Conversely, time from starting therapy was associated with a minimal (0.03%) increase in C min in our patient population. The presence of AML, HSCT and mucositis of any grade were not found to be significantly associated with posaconazole exposure in our patient cohort.
Table 2.
Univariate and multivariate mixed‐effect linear regression analysis of clinical variables associated with posaconazole tablet C min (n = 176)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Unstandardized β‐coefficient (95% CI) | P | Unstandardized β‐coefficient (95% CI) | P | |
| Age (years) | 0.003 (−0.008, 0.014) | 0.586 | – | – |
| Weight (kg) | −0.003 (−0.009, 0.004) | 0.413 | – | – |
| Male | −0.240 (−0.495, 0.014) | 0.063 | −0.284 (−0.532, −0.036) | 0.025 |
| Dose/kg daily (mg) | 0.082 (−0.020, 0.184) | 0.136 | 0.054 (−0.042, 0.151) | 0.264 |
| Days from starting therapy | 0.002 (0.000, 0.005) | 0.029 | 0.003 (0.000, 0.005) | 0.032 |
| Albumin (g l −1 ) | 0.024 (0.004, 0.045) | 0.021 | 0.013 (−0.007, 0.033) | 0.184 |
| Bilirubin (mg dl −1 ) | −0.033 (−0.205, 0.138) | 0.698 | – | – |
| AML | −0.019 (−0.301, 0.264) | 0.894 | – | – |
| HSCT | 0.182 (−0.067, 0.431) | 0.149 | 0.164 (−0.104, 0.432) | 0.226 |
| Mucositis | 0.036 (−0.246, 0.319) | 0.798 | – | – |
| Co‐treatment with PPI | −0.426 (−0.658, −0.193) | 0.008 | −0.447 (−0.689, −0.204) | 0.008 |
| Co‐treatment with steroids (>0.7 mg kg −1 daily) | −0.349 (−0.633, −0.064) | 0.045 | −0.439 (−0.729, −0.149) | 0.022 |
AML, acute myeloid leukaemia; HSCT, haematopoietic stem cell transplantation; PPI, proton pump inhibitors
Figure 2 compares box and whisker plots of posaconazole C min between patients co‐treated with PPIs (PPIs, n = 113 observations), those co‐treated with intermediate or high dose steroids (steroids, n = 9 observations), those co‐treated with PPIs plus intermediate or high dose steroids (PPI + steroids, n = 20 observations), and those who did not receive these co‐treatments (controls, n = 34 observations). Median posaconazole C min was significantly lower among patients receiving these co‐treatments (P < 0.001). Following post‐hoc analysis, each group of co‐treatment had significantly lower median C min than controls (PPIs + steroids vs. controls, 0.74 vs. 1.52 mg l−1, P < 0.00; steroids vs. controls, 1.01 vs. 1.52 mg l−1, P = 0.003; PPIs vs. controls, 1.10 vs. 1.52 mg l−1, P < 0.001).
Figure 2.
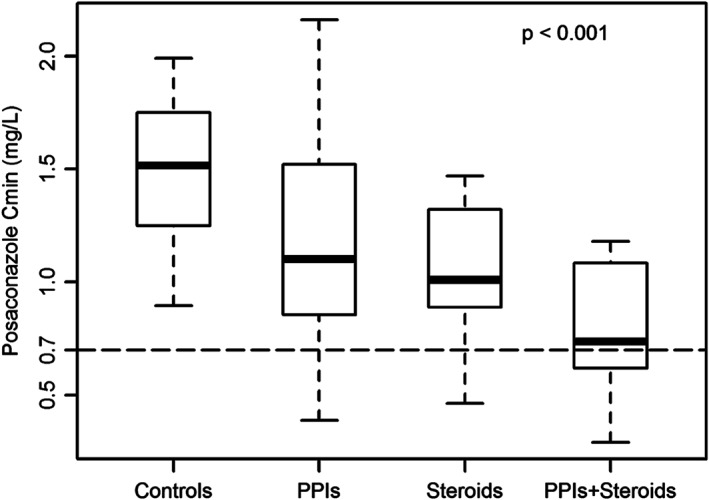
Box (median and 25th–75th percentiles) and whiskers (5th–95th percentiles) plot of posaconazole trough concentrations (C min) following administration of posaconazole tablets in hematologic patients receiving no interacting co‐treatments (Controls; 34 observations in 20 patients), in those receiving proton pump inhibitors (PPIs; 113 observations in 43 patients), in those receiving intermediate or high dose steroids (>0.7 mg kg−1 daily) (Steroids; 9 observations in 5 patients) and in those receiving proton pump inhibitors plus intermediate or high dose steroids (PPIs+ steroids; 20 observations in 13 patients). The dashed line identifies posaconazole target levels for prophylaxis (C min > 0.7 mg l−1) of fungal infections. A P‐value < 0.001 was obtained at Kruskal–Wallis test. A significant difference after post‐hoc Bonferroni correction was observed between Controls and PPIs (P < 0.001), Controls and Steroids (P = 0.003) and Controls and PPIs+ Steroids (P < 0.001)
Discussion
The use of posaconazole is recommended for patients with haematological malignancies who receive induction chemotherapy or for HSCT recipients with GVHD requiring immunosuppression 3. A target C min > 0.7 mg l−1 is advocated 15. Approximately 50% of patients receiving standard dose of the oral suspension (200 mg every 8 h) achieve this target 15. In contrast, higher rates of target attainment are observed with the use of the delayed‐released tablet formulation (70–90%) 7, 20, 21.
Marked variability in posaconazole exposure is still observed in PK studies of the delayed‐released tablet formulation 10, 11, 22, 23, 24. Patients receiving 300 mg daily have a median posaconazole C min concentration of 1.08–1.89 mg l−1 at steady state with a concentration range of <0.1–7.89 mg l−1. Our data are consistent with these observations. We observed a median C min 1.17 mg l−1, with a range of 0.17–4.53 mg l−1 and an overall CV of 50.4%. The extent of inter‐ and intra‐patient variability were 43.9% and 29.3%, respectively, similarly to those previously reported 22. Furthermore, the proportion of subtherapeutic C min is comparable (17.0% in our study vs. 8.6%, 15.4% and 29% in other studies) 10, 23, 25.
Identification of clinical factors associated with posaconazole exposure is of great clinical concern, as posaconazole underexposure was associated with the occurrence of breakthrough IFIs both in experimental animal models 26 and in some clinical studies 5, 27. Although the new delayed‐release formulation seems to be less prone to suboptimal absorption, the pharmacokinetic variability of posaconazole may depend also on other factors that affect CL. Our study shows that the use of PPIs and/or the use of steroids at dosages ≥0.7 mg kg−1 daily are significant risk factors for drug underexposure.
The effects of PPIs on intragastric pH is dose‐dependent and is related to the relative potency of each drug 28. It should be recognized that, differently from what we did in the current study, only a minority of the studies that previously assessed the influence of PPIs on posaconazole tablet exposure specified the type and dosage of the PPI. Different choices and/or different dosages of the PPIs may explain why only some of the real‐world studies on posaconazole tablets still found co‐administration of these drugs as being a risk factor of low posaconazole levels, similarly to us. A retrospective study conducted among 157 patients with haematological malignancies treated with posaconazole tablets showed that at multivariate analysis the use of PPIs (P = 0.015) was a risk factor for subtherapeutic posaconazole concentrations 11. In that study, other risk factors were the presence of diarrhoea (P < 0.001), low baseline albumin concentrations (P = 0.011) and body weight > 90 kg (P = 0.047) 11 . Body weight and diarrhoea were significant risk factors for drug underexposure also in an earlier retrospective investigation 10. Conversely, in a recent retrospective study carried out among 48 haematological malignancy patients who had 325 posaconazole C min measurements, no significant relationship between the use of PPIs and the risk of suboptimal exposure with posaconazole tablets was observed at multivariate analysis 25. Similar findings were documented in another group of haematological patients 20, in lung transplanted patients 29 and in healthy volunteers receiving a 400 mg daily dose 8. The presence of gastro‐intestinal mucositis is not associated with the risk of posaconazole underexposure during the use of delayed‐release tablets in two previous studies 23, 24.
The most novel aspect of our analysis was the finding that corticosteroids may be a risk factor for low posaconazole exposure in patients with haematological malignancy. Posaconazole is metabolized by UGT1A4 12. Intermediate or high dose steroids may have upregulated the activity of this enzyme and resulted in increased CL. This assumption is based on previous studies showing that UGT1A4 may be upregulated by steroids (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1013) in pregnancy leading to an increased elimination of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2622, which is a substrate for UGT1A4 30. UGT1A4 contains http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=606 response elements, which by acting as xenobiotic receptor for a wide range of compounds, including steroids, may induce the glucuronidation process 30, 31. This hypothesis is supported by a retrospective analysis conducted among 52 haematologic patients by Chin et al. 23. These authors found at multivariate analysis that patients not receiving treatment for GVHD [including also high‐dose steroids (either ≥1 mg kg−1 daily for patients with acute GVHD or ≥0.8 mg kg−1 every other day for patients with chronic GVHD] had higher odds of achieving a therapeutic serum level than those receiving GVHD treatment (OR, 5.85; 95% CI, 1.09–31.5; P = 0.04) 23. Indeed, we recognize that collinearity between steroids and HSCT might have prevented HSCT from being identified as an independent risk factor in our study, differently from what was observed by other authors 25.
Overall, our findings suggest that TDM of posaconazole tablet could be especially important for patients receiving both PPI and steroids.
We recognize that our study has several limitations. The retrospective nature and the limited sample size may limit the generalizability of the findings and further prospective studies are required to confirm the correlation with steroids, which only just reached significance. Nevertheless, our work suggests that posaconazole exposure during treatment with delayed‐released tablet formulation may be affected by the use of PPIs and/or of steroids. A prospective clinical study is warranted to confirm our findings.
Competing Interests
W.H. holds or has recently held research grants with F2G, AiCuris, Astellas Pharma, Spero Therapeutics, Matinas Biosciences, Antabio, Amplyx, Allecra, Auspherix and Pfizer, and he holds awards from the National Institutes of Health, Medical Research Council, National Institute of Health Research, FDA and the European Commission (FP7 and IMI). W.H. has received personal fees in his capacity as a consultant for F2G, Amplyx, Ausperix, Spero Therapeutics, Medicines Company, Gilead and Basilea and he is an Ordinary Council Member for the British Society of Antimicrobial Chemotherapy. F.P. has received funds for speaking at symposia organized on behalf of, and has served on scientific advisory boards for, Basilea Pharmaceutica, Gilead Sciences, Merck Sharp & Dohme and Pfizer. The other authors have no competing interests to declare.
Contributors
P.G.C., N.R. and D.L. retrieved and analysed data and contributed to the final manuscript. A.C., W.H., R.F. and F.P. conceptualized the study and wrote the manuscript.
Cojutti, P. G. , Candoni, A. , Lazzarotto, D. , Rabassi, N. , Fanin, R. , Hope, W. , and Pea, F. (2018) Co‐administration of proton pump inhibitors and/or of steroids may be a risk factor for low trough concentrations of posaconazole delayed‐released tablets in adult patients with haematological malignancies. Br J Clin Pharmacol, 84: 2544–2550. 10.1111/bcp.13707.
References
- 1. Lipp HP. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended‐spectrum triazole posaconazole: an overview. Br J Clin Pharmacol 2010; 70: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miceli MH, Churay T, Braun T, Kauffman CA, Couriel DR. Risk factors and outcomes of invasive fungal infections in allogeneic hematopoietic cell transplant recipients. Mycopathologia 2017; 182: 495–504. [DOI] [PubMed] [Google Scholar]
- 3. Mellinghoff SC, Panse J, Alakel N, Behre G, Buchheidt D, Christopeit M, et al Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann Hematol 2018; 97: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cojutti P, Candoni A, Simeone E, Franceschi L, Fanin R, Pea F. Antifungal prophylaxis with posaconazole in patients with acute myeloid leukemia: dose intensification coupled with avoidance of proton pump inhibitors is beneficial in shortening time to effective concentrations. Antimicrob Agents Chemother 2013; 57: 6081–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. Multicenter study of posaconazole therapeutic drug monitoring: exposure‐response relationship and factors affecting concentration. Antimicrob Agents Chemother 2012; 56: 5503–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jang SH, Colangelo PM, Gobburu JV. Exposure‐response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther 2010; 88: 115–119. [DOI] [PubMed] [Google Scholar]
- 7. Cumpston A, Caddell R, Shillingburg A, Lu X, Wen S, Hamadani M, et al Superior serum concentrations with Posaconazole delayed‐release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother 2015; 59: 4424–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kraft WK, Chang PS, van Iersel ML, Waskin H, Krishna G, Kersemaekers WM. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother 2014; 58: 4020–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kersemaekers WM, Dogterom P, Xu J, Marcantonio EE, de Greef R, Waskin H, et al Effect of a high‐fat meal on the pharmacokinetics of 300‐milligram posaconazole in a solid oral tablet formulation. Antimicrob Agents Chemother 2015; 59: 3385–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 2015; 58: 432–436. [DOI] [PubMed] [Google Scholar]
- 11. Tang LA, Marini BL, Benitez L, Nagel JL, Miceli M, Berglund C, et al Risk factors for subtherapeutic levels of posaconazole tablet. J Antimicrob Chemother 2017; 72: 2902–2905. [DOI] [PubMed] [Google Scholar]
- 12. Ghosal A, Hapangama N, Yuan Y, Achanfuo‐Yeboah J, Iannucci R, Chowdhury S, et al Identification of human UDP‐glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab Dispos 2004; 32: 267–271. [DOI] [PubMed] [Google Scholar]
- 13. Maleki S, Corallo C, Coutsouvelis J, Singh J. Failure to achieve therapeutic levels with high‐dose posaconazole tablets potentially due to enhanced clearance. J Oncol Pharm Pract 2018; 24: 63–66. [DOI] [PubMed] [Google Scholar]
- 14. Dolton MJ, Ray JE, Marriott D, McLachlan AJ. Posaconazole exposure‐response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother 2012; 56: 2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 2014; 69: 1162–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franceschi L, D'Aronco S, Furlanut M. Development and validation of a liquid chromatography‐tandem mass spectrometry method for the determination of voriconazole and posaconazole in serum samples. J Bioanal Biomed 2011; 3: 92–97. [Google Scholar]
- 17. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander SP, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174 (Suppl 1): S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexander SP, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 2017; 174 (Suppl 1): S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pham AN, Bubalo JS, Lewis JS 2nd. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 2016; 59: 226–233. [DOI] [PubMed] [Google Scholar]
- 21. Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jimenez JL, et al Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother 2016; 71: 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boglione‐Kerrien C, Picard S, Tron C, Nimubona S, Gangneux JP, Lalanne S, et al Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol 2018; 144: 127–134. [DOI] [PubMed] [Google Scholar]
- 23. Chin A, Pergam SA, Fredricks DN, Hoofnagle AN, Baker KK, Jain R. Evaluation of posaconazole serum concentrations from delayed‐release tablets in patients at high risk for fungal infections. Antimicrob Agents Chemother 2017; 61: pii: e00569‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanstraelen K, Prattes J, Maertens J, Lagrou K, Schoemans H, Peersman N, et al Posaconazole plasma exposure correlated to intestinal mucositis in allogeneic stem cell transplant patients. Eur J Clin Pharmacol 2016; 72: 953–963. [DOI] [PubMed] [Google Scholar]
- 25. Lenczuk D, Zinke‐Cerwenka W, Greinix H, Wolfler A, Prattes J, Zollner‐Schwetz I, et al Antifungal prophylaxis with posaconazole delayed‐release tablet and oral suspension in a real‐life setting: plasma levels, efficacy, and tolerability. Antimicrob Agents Chemother 2018; 62: pii: e02655‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, Cacciapuoti A, et al Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob Agents Chemother 2004; 48: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft‐versus‐host disease. Pharmacotherapy 2007; 27: 1627–1636. [DOI] [PubMed] [Google Scholar]
- 28. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al Relative potency of proton‐pump inhibitors‐comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009; 65: 19–31. [DOI] [PubMed] [Google Scholar]
- 29. Launay M, Roux A, Beaumont L, Douvry B, Lecuyer L, Douez E, et al Posaconazole tablets in real‐life lung transplantation: impact on exposure, drug–drug interactions, and drug management in lung transplant patients, including those with cystic fibrosis. Antimicrob Agents Chemother 2018; 62: pii: e02061‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up‐regulation of UDP‐glucuronosyltransferase (UGT) 1A4 by 17beta‐estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos 2009; 37: 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 2002; 23: 687–702. [DOI] [PubMed] [Google Scholar]


