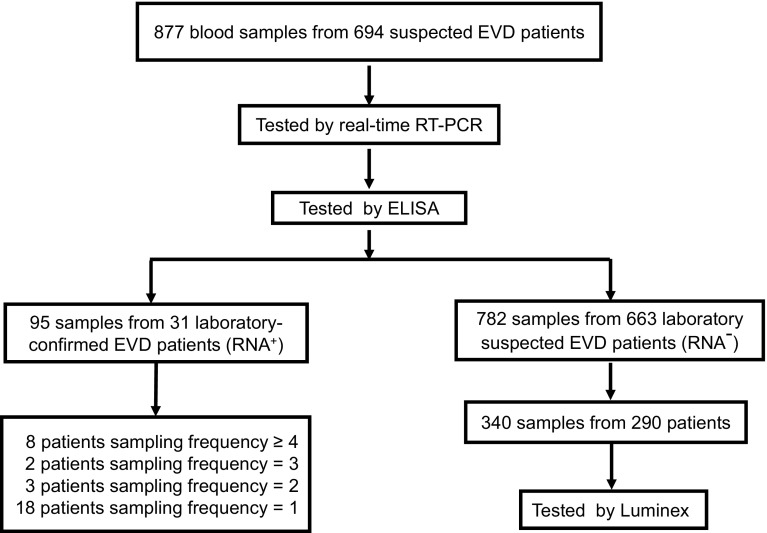
Fig. 1.
A schematic representation of the study methodology. In total, 877 whole blood specimens from 694 suspected patients were first assessed for the presence of Ebola virus (EBOV) RNA via real-time polymerase chain reaction assays, and IgM and IgG titers were determined using an enzyme-linked immunosorbent assay for EBOV-specific human IgM/IgG thereafter. Thirty-one patients were confirmed as EVD patients, from whom 95 samples were obtained at different sampling frequencies, as shown. Among the remaining 782 samples from 663 RNA-negative suspected EVD patients, 340 samples from 290 patients were randomly selected to be assessed via Luminex for IgM and IgG detection.