Figure 3: Summary of Side Effects in Major Recent Trials of SGLT2-i and GLP-1 RA.
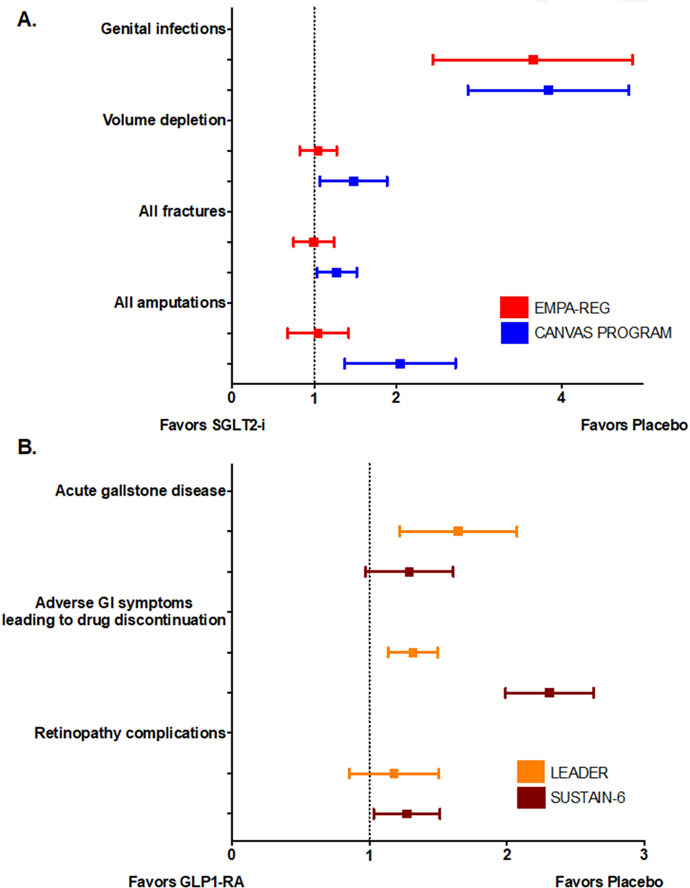
GI, gastrointestinal; GLP-1RA, glucagon-like peptide receptor agonist; SGLT2-i, sodium-glucose cotransporter 2 inhibitor. Panel A. Side effects of SGLT2-i. Genital infections were significantly increased with SGLT-2i use in EMPA-REG OUTCOME and the CANVAS Program. Amputation risk was significantly increased with canagliflozin use. Panel B. Side effects of GLP-1 RA. Liraglutide and semaglutide were significantly associated with higher drug discontinuation rates due to adverse GI symptoms. Acute gallstone disease was significantly increased with liraglutide use and retinopathy complication was significantly increased with semaglutide use.
