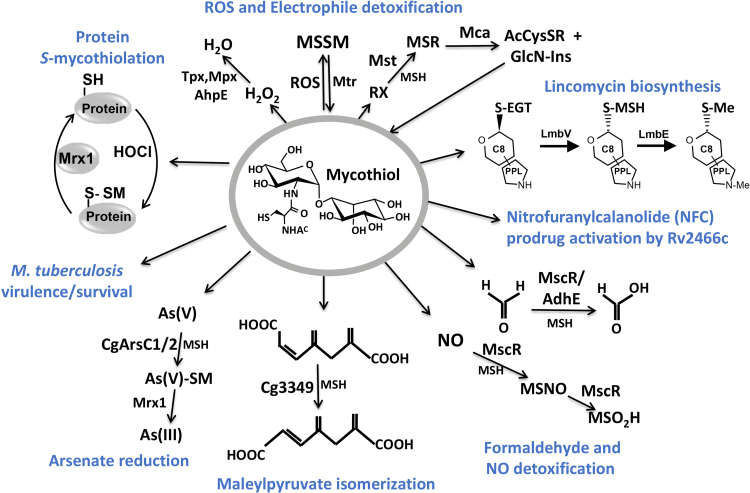
Fig. 4.
The functions of mycothiol (MSH) in Actinomycetes. Mycothiol (MSH) is oxidized by ROS to mycothiol disulfide (MSSM) and regenerated by the mycothiol disulfide reductase (Mtr). The antioxidant enzymes Mpx, Tpx and AhpE were shown to function in H2O2 detoxification. Electrophiles (RX) are detoxified by the MSH S-transferase (MST) leading to MS-electrophiles (MSR) that are cleaved by the MSH S-conjugate amidase (Mca) to mercapturic acids (AcCyS-R) that are exported. MSH and ergothioneine (EGT) are involved in natural product biosynthesis of the lincosamide antibiotic lincomycin in Streptomyces lincolnensis. MSH functions as cofactor for the nitroreductase Rv2466c which activates the new mycobactericidal prodrug Nitrofuranylcalanolide (NFC) for reduction of the nitro group to amines. MSH is also a thiol-cofactor for detoxification of formaldehyde, S-nitrosomycothiol (MSNO) and arsenate. MscR is involved in MSNO detoxification generating MSH sulfinamide (MSONH2). MscR and AdhE both catalyze oxidation of formaldehyde to formate. In C. glutamicum, MSH is a cofactor for maleylpyruvate isomerase involved in isomerization of maleylpyruvate to fumaryl pyruvate. The arsenate reductases ArsC1/C2 catalyze conjugation of MSH to arsenate [Ars(V)] which is further reduced by Mrx1 to arsenite [Ars(III)]. MSH is also important for growth, survival and virulence in M. tuberculosis. Under HOCl stress, MSH functions in post-translational modification of proteins, termed as S-mycothiolations. This figure is adapted from [2].