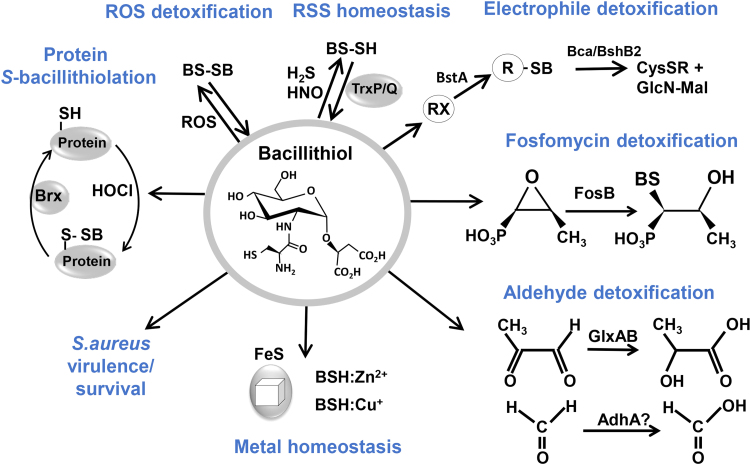
Fig. 7.
The many functions of bacillithiol in Firmicutes. BSH is involved in detoxification of ROS, RES, HOCl, RSS and antibiotics (fosfomycin, rifampicin) in B. subtilis and S. aureus. ROS lead to oxidation of BSH producing bacillithiol disulfide (BSSB). Under sulfide stress, BSH persulfides (BSSH) were determined, which can be reduced by thioredoxins (TrxP and TrxQ). Electrophiles (RX) are conjugated to BSH by the BSH S-transferase BstA to form BS-electrophiles (BSR). The BSH S-conjugate amidases Bca or BshB2 cleave BSR into GlcNAc-Mal and mercapturic acids (CysSR) that are exported. BSH is used as cofactor for the epoxide hydrolase FosB in fosfomycin detoxification and functions as a cofactor for the glyoxalases GlxA and GlxB in B. subtilis in methylglyoxal detoxification. GlxA converts BSH-hemithioacetal to S-lactoyl-BSH that is a substrate for GlxB producing D-lactate. BSH-dependent detoxification of formaldehyde might be catalyzed by the formaldehyde dehydrogenase (AdhA) in B. subtilis. BSH functions in metal homeostasis as Zn2+ buffer and in FeS cluster assembly. In S. aureus, BSH is important for the virulence of S. aureus in macrophage infection assays. Under HOCl stress, proteins are S-bacillithiolated, which can be reversed by bacilliredoxins (Brx). This figure is adapted from [2].