Summary
In the case of organ transplantation accompanied by vascular anastomosis, major histocompatibility complex mismatched vascular endothelial cells become a target for graft rejection. Production of a rejection-free, transplantable organ, therefore, requires simultaneous generation of vascular endothelial cells within the organ. To generate pluripotent stem cell (PSC)-derived vascular endothelial cells, we performed blastocyst complementation with a vascular endothelial growth factor receptor-2 homozygous mutant blastocyst. This mutation is embryonic lethal at embryonic (E) day 8.5–9.5 due to an early defect in endothelial and hematopoietic cells. The Flk-1 homozygous knockout chimeric mice survived to adulthood for over 1 year without any abnormality, and all vascular endothelial cells and hematopoietic cells were derived from the injected PSCs. This approach could be used in conjunction with other gene knockouts which induce organ deficiency to produce a rejection-free, transplantable organ in which all the organ's cells and vasculature are PSC derived.
Keywords: tissue regeneration, vascular endothelial cells, hematopoietic cells, blastocyst complementation, pluripotent stem cells
Graphical Abstract
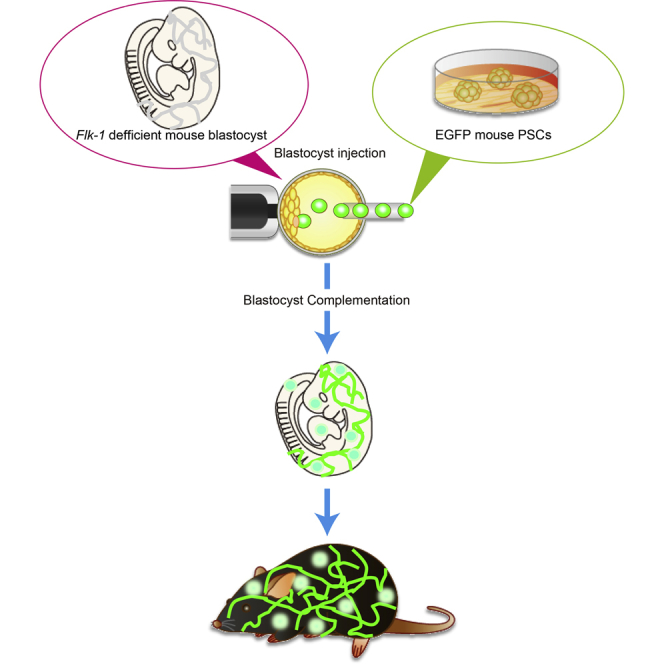
Highlights
-
•
Flk-1-deficient PSCs did not contribute to vascular endothelial cells in chimeric mice
-
•
Flk-1-deficient mice survived into adulthood by blastocyst complementation
-
•
Both vascular endothelial cells and hematopoietic cells were generated from PSCs
In this article, Yamaguchi and colleagues generated vascular endothelial cells and hematopoietic cells from pluripotent stem cells in vivo by blastocyst complementation technique. This approach could be used to produce a rejection-free, transplantable organ in which all the organ's cells and vasculature are pluripotent stem cell derived.
Introduction
Two key constraints to successful treatment using human organ transplantation are graft rejection and organ shortage. Autologous tissue stem cells or cell sheets generated from patient-derived induced pluripotent stem cells (iPSCs) have been transplanted to eliminate rejection-associated problems. Unfortunately, these cell therapies are often not adequate to repair or replace a failing organ (Assawachananont et al., 2014, Takebe et al., 2013).
Blastocyst complementation can be used to generate entire organs derived from pluripotent stem cells (PSCs). In this method, PSCs are injected into a blastocyst that has been genetically modified to prevent development of a targeted organ (Kobayashi et al., 2010, Matsunari et al., 2013, Usui et al., 2012, Yamaguchi et al., 2017). The PSCs integrate into the growing embryo to form a chimeric animal; however, the targeted organ develops exclusively from the injected PSCs.
We first successfully generated PSC-derived organs using blastocyst complementation in 2010. To target the pancreas we knocked out Pdx1, resulting in apancreatic mice with severe hyperglycemia that died within a week after birth. We rescued this phenotype by injecting mouse or rat PSCs into the Pdx1 knockout (KO) mouse blastocysts. Nearly all pancreatic cells, including exocrine and endocrine cells, were derived from the injected PSCs. However, cells originating from non-pancreatic lineages, such as blood vessels and stromal cells, were chimeric for both blastocyst-derived cells and PSC-derived cells (Kobayashi et al., 2010). We had similar results when targeting the kidney with blastocyst complementation—the renal lineage cells were derived from injected PSCs, whereas non-renal lineages within the kidneys were chimeric (Usui et al., 2012).
A major histocompatibility complex (MHC) mismatch of the vascular endothelial cells (a monolayer of cells lining the lumen of vessels) will elicit hyperacute rejection against the blood vessel endothelium in the transplanted organ. Hyperacute rejection often occurs within 24 hr and is initiated by recipient's natural antibodies against the antigens present in the graft's vascular endothelial cells. After recognition of the antigens, the complement and coagulation systems are activated, resulting in inflammation and vascular occlusion. This will cause the graft to rapidly necrose. Between 6 days and 3 months after transplantation, acute rejection may occur, which is also caused by an MHC mismatch of the vascular endothelial cells. Acute rejection caused by effector T cells, antibodies, and activated T cells will directly lyse the graft's vessels and produce cytokines that recruit and activate inflammatory cells (Platt et al., 1990, Platt et al., 1991). Therefore, in the context of blastocyst complementation, it is necessary to generate organs together with vascular endothelial cells in the blood vessels from a patient's iPSCs to prevent organ rejection.
In this study, we aimed to generate blood vessels containing entirely PSC-derived vascular endothelial cells by blastocyst complementation. In mice, vasculogenesis is initiated from the yolk sac blood islands at E7.5 and is dependent on several key factors. Disrupting vascular endothelial growth factor receptor 2 (VEGFR2/Flk-1/KDR) inhibits vasculogenesis due to impaired endothelial and hematopoietic cell development, resulting in embryonic lethality around E9.0 (Shalaby et al., 1995, Shalaby et al., 1997). Since Flk-1 mutant mice (Flk-11173F/1173F) represent the same phenotype as a Flk-1 KO mice, Flk-1 mutant blastocysts were used as our host embryo for blastocyst complementation (Sakurai et al., 2005).
Results
Flk-11173F/1173F miPSC-Derived Cells Cannot Contribute to PECAM1-Expressing Vascular Endothelial Cells in Adult Mice
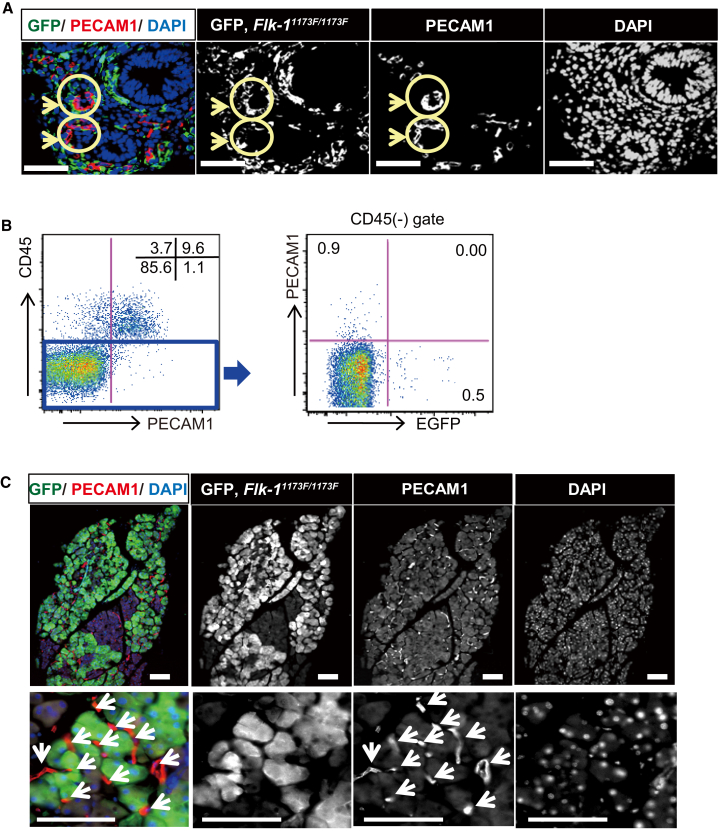
Although the Flk-1 homozygous mutant (or Flk-11173F/1173F) mouse is well studied at early embryonic stages, because of embryonic lethality at E8.5–9.5, the role of Flk-1 in vasculogenesis from E9.5 to adulthood is unclear. To address this issue, we generated chimeric mice by injecting enhanced green fluorescent protein (EGFP)-marked Flk-11173F/1173F mouse-induced PSCs (miPSCs) into wild-type (WT) mouse blastocysts (Figures S1A and S1B). We first analyzed the contribution of Flk-11173F/1173F cells to blood vessels in E13.5 embryos (Figures 1A and 1B). The immunofluorescent staining of a section of intestine with relatively high chimerism revealed that the EGFP-expressing Flk-11173F/1173F iPSC-derived cells did not express platelet endothelial cell adhesion molecule 1 (PECAM1) (arrow) (Figure 1A). In addition, flow cytometric analysis of fetal liver showed that the CD45− and PECAM1+ (also known as CD31) vascular endothelial cells did not express EGFP (Figure 1B). Next, in order to analyze the contribution of Flk-11173F/1173F iPSCs in adult chimeric mice, we performed immunofluorescent analysis of a pancreas that showed relatively high chimerism and found that EGFP+ Flk-11173F/1173F iPSC-derived cells did not express PECAM1 (Figures 1C, S1C, and S1D).
Figure 1.
Phenotype of Vasculogenesis in Flk-11173F/1173F iPSC-Derived Chimeric Mice
(A) Immunohistological analysis of vascular endothelial cells in embryo of Flk-11173F/1173F iPSC-derived chimeric mouse at E13.5. Sections were stained with antibodies against GFP for Flk-11173F/1173F iPSC-derived cells, and PECAM1 for endothelial cells, and cell nuclei were stained with DAPI. The vascular endothelia are indicated (arrows).
(B) Flow cytometry analysis of vascular endothelial cells in fetal liver. Fetal liver cells were stained with antibodies against CD45 and PECAM1. Representative results from n = 8 independent experiments are shown.
(C) Immunohistological analysis of sections obtained from pancreas. Sections were stained with antibodies against GFP for Flk-11173F/1173F iPSC-derived cells, antibodies against PECAM1 for endothelial cells (arrows) and DAPI for nuclear counterstaining. Lower panels show higher magnification.
Scale bars: 50 μm (A) and 100 μm (C).
These results indicate that Flk-11173F/1173F iPSC-derived cells cannot contribute to vasculogenesis or angiogenesis from the early embryo to adulthood. Thus, the Flk-11173F/1173F mouse is a suitable host animal for blastocyst complementation when generating PSC-derived blood vessels.
mPSCs Can Rescue Flk-1 KO Lethality by Blastocyst Complementation
To generate blood vessels in Flk-11173F/1173F mice, blastocysts and morulae obtained from an intercross of Flk-1+/1173F mice were injected with EGFP or KuO-labeled miPSCs or mouse embryonic stem cells (mESCs). A total of 105 chimeric mice were born and matured to adults with no remarkable abnormalities. Of these, 11 were Flk-11173F/1173F, indicating that mPSCs can contribute to vasculogenesis and rescue the Flk-1 KO phenotype (Table 1).
Table 1.
Generation of Flk-11173F/1173F Chimera Mice by Blastocyst Complementation
| Injected Cell Lines | No. (%) of Offsprings |
Flk-1 Genotype (%) |
||||
|---|---|---|---|---|---|---|
| Alive | Chimeras | +/+ | +/1173F | 1173F/1173F | NDa | |
| GT3.2 | 48 | 37 (77) | 13 (35) | 20 (54) | 4 (11) | 0 (0) |
| KuOmiPS | 20 | 13 (65) | 9 (69) | 3 (23) | 1b (8) | 0 (0) |
| SGE2 | 56 | 55 (98) | 14 (25) | 18 (33) | 6 (11) | 17 (31) |
| Total | 124 | 105 (85) | 36 (34) | 41 (39) | 11 (11) | 17 (16) |
Not determined.
Neonate.
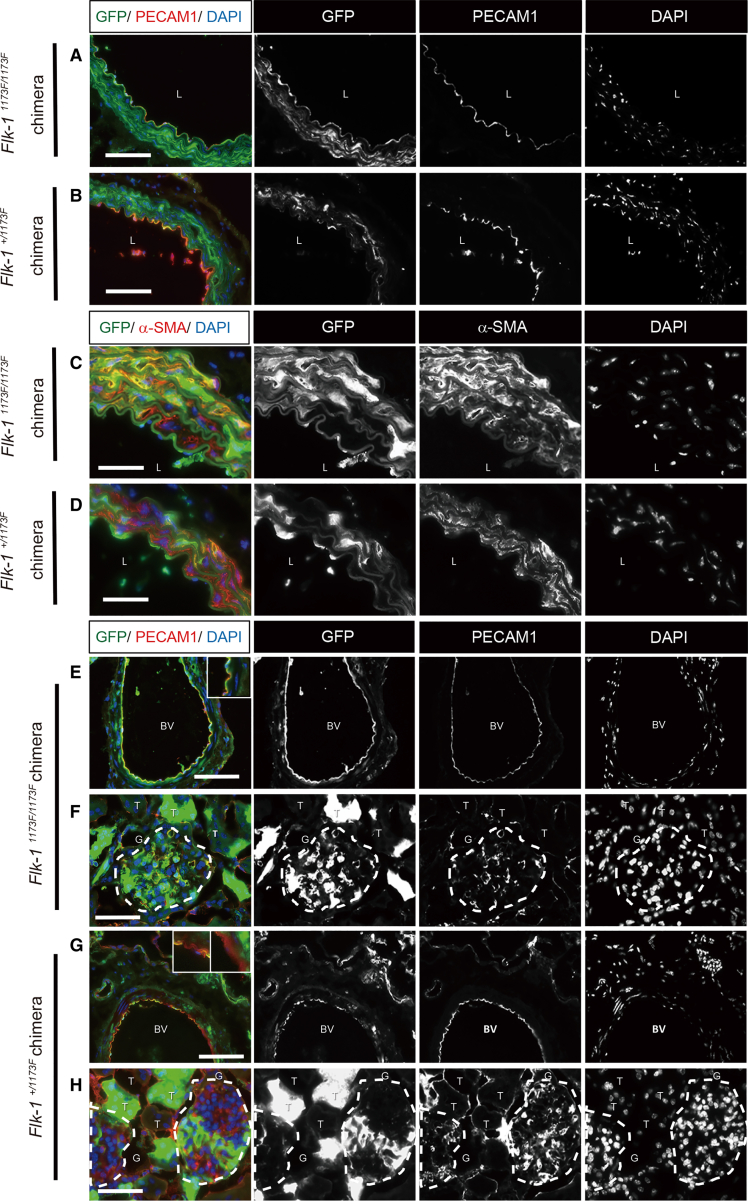
Immunofluorescence was used to analyze the distribution of miPSC-derived cells in each tissue. In the Flk-11173F/1173F chimeric mice, PECAM1+ vascular endothelial cells in the aorta, kidney, lung, and heart were entirely derived from miPSCs. Other structures not of the vascular endothelial lineage, such as vascular smooth muscle in the aorta, Bowman's capsule and renal tubule in the kidney, bronchioles and alveoli in the lung, and myocardium in the heart, were a composite of host and donor derivatives (Figures 2A, 2C, 2E, 2F, S2A, S2B, and S2E). In expected contrast, all structural components, including vascular endothelial cells of the aorta, kidney, lung, and heart in Flk-1+/1173F chimeric mice, were a composite of host and donor derivatives (Figures 2B, 2D, 2G, 2H, S2C, S2D, and S2F).
Figure 2.
Immunohistological Study of Chimeric Mice at Adult Stage
(A–D) The distribution of iPSC-derived cells in sections of aorta and kidney (E–H). Sections were stained with antibodies against GFP, PECAM1, α-SMA, and DAPI. L, lumen; BV, blood vessel; G, glomerulus; T, tubules. Scale bars: 50 μm (C, D, F, and H) and 100 μm (A, B, E, and G).
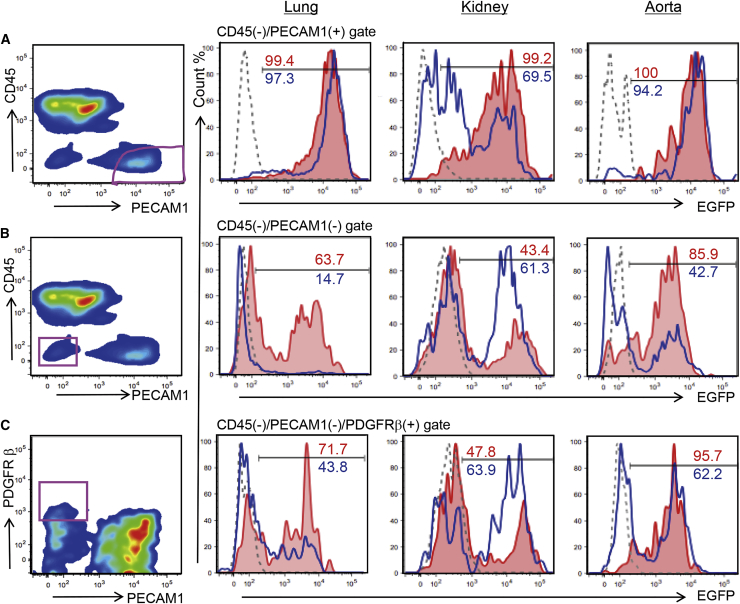
To further analyze the contribution of mPSCs to vascular endothelial cells, we performed fluorescence-activated cell sorting (FACS) analysis of enzymatically dissociated tissues from adult chimeric mice.
In Flk-11173F/1173F chimeric mice, almost 100% of the vascular endothelial cell population (CD45−/PECAM1+) of the lungs, kidneys, and aortas were EGFP positive (Figures 3A, S3A, and S3B), whereas populations other than vascular endothelial cells (CD45−/PECAM1− population), the cells in the vessel wall, or the perivascular cells (CD45−/PECAM1−/platelet-derived growth factor receptor beta [PDGFR β+]) and the overall cells in the lungs, kidneys, and aortas were chimeric (39.8%–95.7%) (Figures 3B, 3C, S3A, and S3B).
Figure 3.
Flow Cytometry Analysis of the EGFP-Expressing, miPSC-Derived Cells in the Vascular Endothelium and Pericytes of Each Organ
Ratio of donor cell contribution in lung, kidney and aorta of Flk-11173F/1173F or Flk-1+/1173F chimeric mice were analyzed using antibodies against CD45, PECAM1, and PDGFRβ.
(A) Vascular endothelial cells (CD45−/PECAM1+) were gated and analyzed for EGFP expression.
(B) Non-vascular endothelial cells (CD45−/PECAM1−) were gated and analyzed for EGFP expression.
(C) Pericytes (CD45−/PECAM1−/PDGFRβ+) were gated and analyzed for EGFP expression.
Red line, Flk-11173F/1173F chimeric mouse; blue line, Flk-1+/1173F chimeric mouse; gray dashed-line, C57BL/6 mouse shown as a control. Three biological replicates were performed.
These results indicate that the mPSCs could complement the vasculogenic niche and create functional blood vessels in Flk-11173F/1173F mice. Moreover, the vascular endothelial cells in blood vessels were completely derived from mPSCs, whereas the other components were composed of host and donor-derived cells. This means whole chimera mouse body was not composed of almost 100% donor cells and successfully complemented specific tissue by blastocyst complementation. Although we tried to generate rat vascular endothelial cells in mice by injecting rat PSCs into Flk-11173F/1173F mouse blastocysts, we could not obtain live Flk-11173F/1173F fetuses at E13.5 and beyond. However, at E9.5, live Flk-11173F/1173F interspecies chimeric fetuses were found at Mendelian ratios (Table S1).
All Hematopoietic Stem Cells in the Flk-11173F/1173F Chimeric Mouse Were Generated from Donor mPSCs
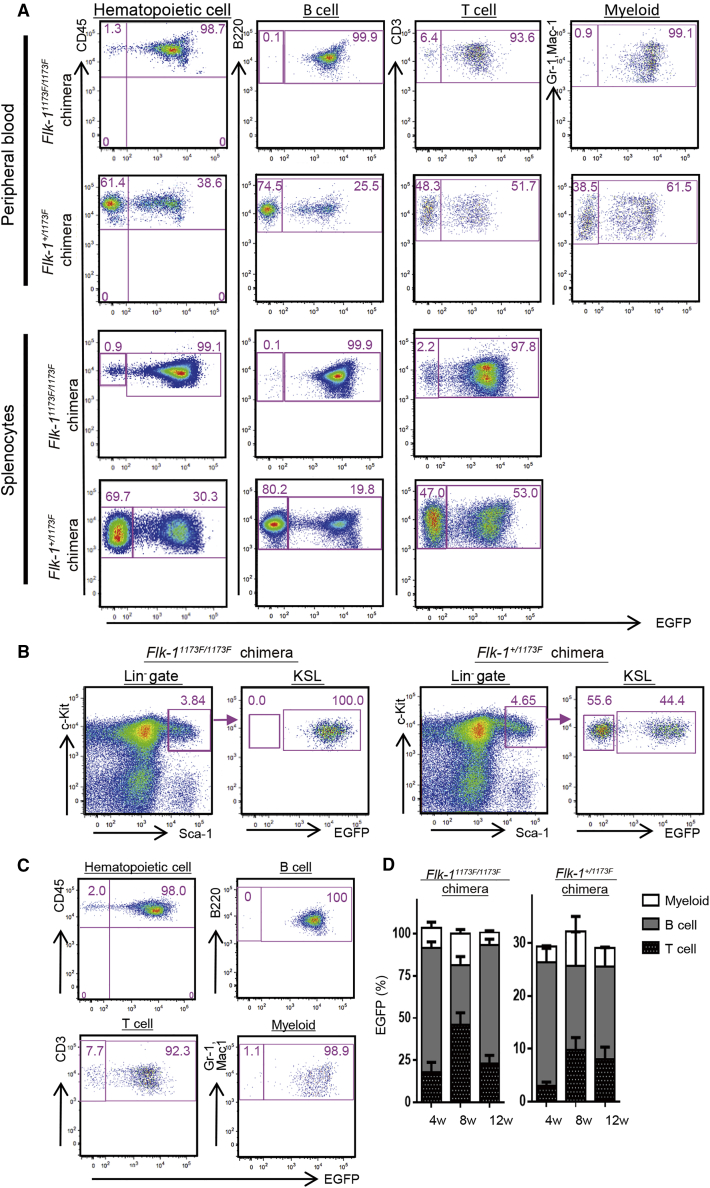
It was previously reported that the Flk-11173F/1173F mouse has an embryonic lethal phenotype due to not only impaired vasculogenesis, but also deficient primitive hematopoiesis at E8.5 to E9.5. Therefore, we analyzed the contribution of mPSCs in CD45+ hematopoietic cells to confirm whether the hematopoietic cells are also completely derived from injected mPSCs in Flk-11173F/1173F chimeric mice. FACS analysis of peripheral blood mononuclear cells (PBMCs) revealed that all lineages of hematopoietic cells were almost 100% EGFP positive in Flk-11173F/1173F chimeric mice, whereas hematopoietic cells in Flk-1+/1173F chimeric mice were chimeric. Similar results were obtained by the FACS analysis of splenocytes (Figures 4A, S4A, and S4B). We also analyzed the percentage of EGFP+ cells in, c-Kit+, Sca-1+, and lineage− (KSL) hematopoietic progenitor and stem cells. We found that the chimerism of EGFP+ cells in the KSL population was 100% in Flk-11173F/1173F chimeric mice and chimeric in Flk-1+/1173F chimeric mice (Figures 4B, S4C, and S4D). Furthermore, to analyze the reconstitution ability of miPSC-derived hematopoietic stem cells (HSCs) generated in Flk-11173F/1173F chimeric mice, we performed transplantation of the Flk-11173F/1173F chimera's whole bone marrow to sub-lethally irradiated recipient mice (n = 4) and analyzed the engraftment rate. The transplanted cells differentiated into three hematopoietic lineages, including B cell, T cell, and myeloid cells. The percentage of EGFP+ cells in hematopoietic cells was 98%; in B cells was 100%; in T cells was 92.3%; and in myeloid cells was 98.9% (Figures 4C and 4D).
Figure 4.
Flow Cytometry Analysis of Chimerism in miPSC-Derived Hematopoietic Cells
(A) Representative flow cytometric plots of EGFP and hematopoietic lineage marker expression in peripheral blood and splenocytes from Flk-1 mutant chimeras. Three biological replicates were performed.
(B) Representative flow cytometric plots and gating for the c-Kit+Sca1+Lineage− (KSL) fraction within the BM of Flk-1 mutant chimeras. Three biological replicates were performed.
(C) Representative percentage of peripheral blood chimerism of 12 weeks after Flk-1 mutant chimera's BM transplantation into lethally irradiated four recipient mice.
(D) Time course of engraftment of complemented hematopoietic cells are shown. EGFP expression was analyzed in the peripheral blood 4, 8, and 12 weeks after transplantation.
Results are means ± SD of n = 4 (Flk-11173F/1173F; 4 weeks), n = 3 (Flk-11173F/1173F; 8 weeks), n = 2 (Flk-11173F/1173F; 12 weeks), n = 3 (Flk-1+/1173F; 4 weeks), n = 3 (Flk-1+/1173F; 8 weeks), and n = 3 (Flk-1+/1173F; 12 weeks) independent experiments.
These results indicate that mPSCs can complement both the hematopoietic and vasculogenic niche, generating entirely mPSC-derived HSCs and hematopoietic cells in Flk-11173F/1173F chimeric mice by blastocyst complementation.
Discussion
Here we successfully used blastocyst complementation to generate mPSC-derived vascular endothelial cells and hematopoietic cells, including HSCs.
In order to generate Flk-11173F/1173F chimeric mice, fluorescently labeled WT mPSCs were injected into blastocysts/morulae obtained by intercross of a Flk-1+/1173F male with a Flk-1+/1173F female mouse. The birth rate of Flk-11173F/1173F chimeric mice was distorted from Mendelian expectations. This distortion was also observed in E13.5 to E15.5 chimeric fetuses (4/90 = 4.4%). We previously found that the birth rate of Pdx1−/− chimeric mice obey Mendel's law (Kobayashi et al., 2010), suggesting that complementation efficiency may depend on the targeted gene. For example, although the size of the pancreas may be restricted by the number of cells present in the pancreatic anlage, we previously observed that a small pancreas is enough to maintain blood glucose level (data not shown), indicating that the contribution rate of PSCs to pancreatic anlage might not affect the birth rate of complemented mice. On the other hand, Flk-1 is important for the development of multiple tissues that are dispersed throughout the whole body, including vascular endothelial cells, hematopoietic cells, and smooth muscle cells. Hence, the contribution rate of PSCs to the vascular endothelial cell anlage might affect the survival of the developing mouse.
Although we successfully generated entirely PSC-derived vascular endothelial cells, the vessel wall and the perivascular cells, which include smooth muscle cells, pericytes, and renal mesangial cells, were chimeric because those tissues can develop from both host neural crest and Flk-1+ mesoderm cells (Hirschi and Majesky, 2004, Nakamura et al., 2006).
When performing blastocyst complementation with Flk-11173F/1173F mouse embryos, all hematopoietic cells were entirely derived from the injected mPSCs. Primitive hematopoiesis is initiated in the yolk sac blood island at E7.5. The site of hematopoiesis is then migrated to the aorta-gonad-mesonephros (AGM) region, after which definitive hematopoiesis is initiated around E10.5. Previous studies showed that HSCs first emerge from vascular endothelial cells in the dorsal aorta at E10.5 (Bertrand et al., 2010, Boisset et al., 2010, Herbert et al., 2009, Kissa and Herbomel, 2010). When performing blastocyst complementation with Flk-11173F/1173F mouse embryos, the HSCs likely emerged from the complemented vascular endothelial niche, resulting in generation of both PSC-derived vascular endothelial cells and hematopoietic cells. Because residual hematopoietic cells left in a graft might induce graft versus host disease (GVHD) after transplantation, simultaneous generation of tissue and hematopoietic cells from PSCs might further reduce the risk of GVHD.
Although most of the PBMCs from Flk-11173F/1173F chimeric mice were EGFP positive, FACS analysis revealed that 0.2% to 1.3% of hematopoietic cells were EGFP negative. We found that the HSCs in the bone marrow of vascular system complemented Flk-11173F/1173F chimera mouse were 100% EGFP positive. Moreover, EGFP-negative hematopoietic cells were also observed in peripheral blood of the EGFP transgenic mouse, which is the origin of donor PSCs. These results indicate that gene silencing had occurred in EGFP-negative hematopoietic cells in peripheral blood of rescued Flk-11173F/1173F chimera mice. In addition, there is another possibility that EGFP-negative hematopoietic cells were derived from the origin other than HSCs, as previously described (Ginhoux et al., 2010, Hoeffel et al., 2012).
Because our goal is to generate tissues or organs in a xenogenic animal, we also tried to make rat vascular endothelial cells and hematopoietic cells in mice by injecting rat iPSCs into mouse blastocysts obtained by an intercross of Flk-1+/1173F mice. Because Flk-11173F/1173F mice were embryonic lethal at E8.5∼E9.5, even the incomplete complemented embryos were viable until E9.5 and follow Mendelian laws. Because incomplete complementation leads to immature vascularization and embryonic death after E9.5, the birth rate of Flk-11173F/1173F chimeric mice and survival rate of E13.5–E15.5 chimeric fetuses were distorted from Mendelian expectations in both intraspecies complementation and interspecies complementation setting. The incomplete contribution of donor cells to the AGM region can induce embryonic death because the ischemic condition is caused by hematopoietic cell and vascular deficiency.
Although mPSCs can rescue the Flk-11173F/1173F phenotype in mouse blastocysts, efficiency is low. Efficiency could be immediately increased by abandoning the heterozygous intercross approach, which results in only 25% Flk-11173F/1173F blastocysts. Instead, adopting nuclear transfer technology or utilizing Flk-1-driven suicide genes would result in 100% Flk-1-deficient embryos. These approaches are also useful for performing multiple gene disruptions.
Currently, organs transplanted into human patients are supplied by human donors. These allogeneic grafts may be rejected and require each recipient to endure lifelong immunosuppression. By simultaneously disrupting Flk-1 and genes required for organogenesis (e.g, Pdx1 for pancreas), autologous organs could be generated from patient-specific iPSCs via interspecies blastocyst complementation. These iPSC-derived organs would not be contaminated with xenogenic cells in the vascular endothelium, eliminating rejection-associated problems (Platt et al., 1990, Platt et al., 1991). Chimeric animal models generated from Flk-1 deficient blastocysts thus provide a key step toward in vivo generation of rejection-free organs for use in regenerative medicine.
Experimental Procedures
Animals
C57BL/6NCrSlc, BDF1, ICR mice were purchased from SLC Japan (Shizuoka, Japan). Flk-1+/1173F mice were kindly provided by Dr. M. Shibuya (University of Tokyo, Tokyo Medical and Dental University, and Jobu University) (Sakurai et al., 2005), and were crossed with C57BL/6 or BDF1 mice. All experiments were performed in accordance with the animal care and use committee guidelines of the Institute of Medical Science, University of Tokyo.
Culture of iPSCs/ESCs
Undifferentiated mouse iPSCs (GT3.2(XY) (Kobayashi et al., 2010), mESCs (SGE2) derived from EGFP transgenic mice, Flk-11173F/1173F miPSCs, and Kusabira Orange (KuO) miPS) were maintained on mitomycin C-treated mouse embryonic fibroblast (MEF) feeder cells in Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO) supplemented with 15% fetal bovine serum (Nichirei Bioscience, Tokyo, Japan), 0.1 mM 2-mercaptoethanol (Invitrogen, San Diego, CA, USA), 1% nonessential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 1% L-glutamine penicillin streptomycin (Sigma), and 1,000 U/mL of mouse leukemia inhibitory factor (LIF; Millipore, Bedford, MA, USA).
Flk-11173F/1173F miPSCs were generated from embryonic fibroblasts harvested from an E13.5 Flk-11173F/1173F chimeric mouse fetus (injected with GT3.2 iPSCs at the blastocyst stage). The iPSCs were generated by introducing three reprograming factors (Oct-3/4, Sox-2, and Klf-4) and EGFP in all-in-one inducible lentiviral vector (Ai-LV) (Yamaguchi et al., 2012).
KuO miPS were generated from MEFs of KuO mice by introducing three reprograming factors (Oct-3/4, Sox-2, and Klf-4) in Ai-LV and CAG huKO IRES Puro (Hamanaka et al., 2013).
riPST1-3 were generated from rat embryonic fibroblasts of Wistar rat by introducing three reprograming factors (Oct-3/4, Sox-2, and Klf-4) in Ai-LV (Hamanaka et al., 2011). Fri6.1 were generated from rat embryonic fibroblasts of F344 rat by introducing three reprograming factors (Oct-3/4, Sox-2, and Klf-4) in pMx mOKS IRES tdTomato and CAG huKO IRES Puro.
Maintenance of rat iPSCs has been previously described (Hamanaka et al., 2011). Briefly rat iPSCs were maintained on mitomycin C-treated MEF feeder cells in N2B27 medium (Invitrogen) supplemented with 1,000 U/mL mouse LIF (Millipore, Bedford, MA, USA) or rat LIF (Rat ESGRO; Millipore ESG 2206), 1 μM GSK3 inhibitor CHIR99021 (Axon Medchem),1 μM MEK inhibitor PD0325901 (Stemgent or Wako), 1% L-glutamine penicillin streptomycin (Sigma). All PSC lines were authenticated by chimera formation.
Generation of Chimeric Mice and Genotyping of iPSCs/ESCs Derived from Chimeric Mice
Chimeric mice were generated by injection of miPSCs into 3.5 days post coitum (dpc) blastocysts and mESCs into 2.5 dpc morulae of WT or Flk-1+/1173F intercrossing embryos, followed by transfer into host uteri as previously described (Kobayashi et al., 2010)
For genotyping, chimeric mouse fibroblasts were isolated from E13.5–E14.5 mice and adult mice. GFP mouse fibroblasts were sorted by cytometry using MoFlo or Aria cytometer (Beckman Coulter, Fullerton, CA, USA). Genotypes of mice were confirmed using extracted genomic DNA from sorted GFP-expressing cells. PCR primers for amplification of Flk-1 were forward; 5′-GCCTCTTCCAAGACAGTCT-3′ and reverse; 5′-GAACCTTCAGCAGGTTTCCTATTTG-3′. After BspEI digestion of the PCR products (729 bp), the products from the WT allele were not digested (729 bp), but the products from the Flk-11173F mutant allele were digested into 523 and 206 bp bands.
Histological Analysis
For frozen sections, mouse tissues were fixed with 4% paraformaldehyde, washed with PBS, soaked in 30% sucrose and mounted in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan). Frozen sections were stained immunohistochemically. Each section was incubated with the primary antibody overnight at 4°C and with the secondary antibody for 1 hr at room temperature.
The antibodies used for the primary antibody were antibodies against EGFP (A11122, Invitrogen; and ab6673, Abcam, Cambridge, UK), platelet endothelial cell adhesion molecule-1 (PECAM1, #550274, BD PharMingen, Franklin Lakes, NJ, USA), and α-smooth muscle actin (αSMA, ab5694, Abcam). Secondary antibodies used were Alexa 488-conjugated-goat anti-rabbit IgG (A11034), -donkey anti-rabbit IgG (A21206), -donkey anti-goat IgG (A11055), Alexa 546-conjugated-goat anti-rat IgG (A11081), -donkey anti-rabbit IgG (A10040), Alexa 647-conjugated-goat anti-rabbit IgG (A21245), -chicken anti-rat IgG (A21472) (Invitrogen). After antibody treatment, sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) and mounted with fluorescence mounting medium (Dako, Glostrup, Denmark). The sections were observed under fluorescence microscopy (BZ9000, Keyence, Itasca, IL)
Flow Cytometry Analysis of iPSC/ESC-Derived Chimeras and Bone Marrow Transplantation
Cells were dissociated with accutase (Innovative Cell Technologies, San Diego, CA) for fetal liver, with accutase or trypsin for embryonic fibroblast, and with collagenase IA for adult tissues. Cells were stained with phycoerythrin (PE)-conjugated anti-CD3ɛ (#100307), -CD45.2 (#109807) (BioLegend, San Diego, CA) and -CD31 (PECAM1, #12-0311-81, eBioscience, San Diego, CA), allophycocyanin (APC)-conjugated anti-CD45 (#559864, BD Pharmingen, Franklin Lakes, NJ), -CD45.1 (#17-0453-82, eBioscience) and -CD140β (PDGFRβ, #136008, BioLegend), phycoerythrin cyanine7 (PECy7)-conjugated anti-CD45 (#103113, BioLegend, or #25–0451081, eBioscience), -Gr-1 (#108415) and -Mac-1 (CD11b, #101215), Pacific blue (PB)-conjugated anti-CD45 (#103126) and B220 (#163230) (BioLegend), brilliant violet (BV)-421-conjugated anti-CD31 (#563356, BD Bioscience, San Jose, CA), allophycocyanin cyanine7 (APC-Cy7)-conjugated anti-CD3 (#100221, BioLegend), and purified-CD16/32 (#553142, BD Pharmingen) antibodies, and propidium iodide for removing dead cells. HSCs were stained with APC-conjugated anti-c-Kit (#17-1171-81, eBioscience), PE-conjugated anti-Sca-1 (#108108), PB-conjugated anti-Sca-1 (#108119) (BioLegend), and the lineage antibody-mix consisting of biotinylated anti-Gr-1 (#13593185), -Mac-1 (#13011285), -CD4 (#13004285), -Ter119 (#13592185), -interleukin-7 receptor (#13127185) (eBioscience), and -CD8 (#13008185) and -B220 (#13045285) (BioLegend) antibodies. The biotinylated antibodies were developed with streptavidin-APC-Cy7 (#405208, BioLegend).
After being washed with PBS containing 2% fetal calf serum (FCS), stained cells were analyzed by flow cytometry using Cant II or Aria II equipment (Becton-Dickinson, Franklin Lakes, NJ).
For bone marrow (BM) transplantation, total BM cells were suspended in 2% FCS in PBS; 2 × 107 total BM cells in 200 μL of medium was injected into each of a group of lethally irradiated C57BL/6 mice. Peripheral blood cells of the recipient mice were analyzed 4, 8, and 12 weeks after transplantation.
Statistics and Reproducibility
The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment. Statistical significance was calculated by F test and Student's t test (compare two groups) and the similarity to the Mendelian ratio was analyzed by chi-squared test (with Excel and GraphPad Prism software). p < 0.05 was considered to be statistically significant. Data are presented as mean ± SD.
Author Contributions
S.H. and T.Y. designed this study, and wrote the manuscript. S.H. performed the experiments. A.U., M.K., and H.S. performed embryo manipulation. A.U. and H.M. performed data analysis. N.M., A.Y., T.H., and S.Y. performed animal experiments. T.K. performed establishment of miPSCs. F.P.S wrote the manuscript. H.N. designed and supervised this study, and wrote the manuscript.
Acknowledgments
We thank H. Tsukui and J. Ooehara for technical support, K. Okada for secretarial support, Dr. M. Shibuya for kindly providing Flk-1+/1173F mice and Dr. M. Watanabe for critical advice in preparing the manuscript. This work was supported by grants from Japan Science and Technology Agency, Exploratory Research for Advanced Technology, Leading Advanced Projects for Medical Innovation, AMED under grant number JP18gm0010002, KAKENHI grant number 17K10536, research grant for type 1 diabetes, Japan IDDM network and California Institute for Regenerative Medicine Grant Number LA1-06917. H.N. is a co-founder and shareholder of iCELL Inc., ChimaERA Corporation, and ReproCELL Inc.
Published: September 20, 2018
Footnotes
Supplemental Information includes four figures and one table and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.08.015.
Contributor Information
Tomoyuki Yamaguchi, Email: tomoyama@ims.u-tokyo.ac.jp.
Hiromitsu Nakauchi, Email: nakauchi@stanford.edu.
Supplemental Information
References
- Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., Sasai Y., Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2:662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J.Y., Chi N.C., Santoso B., Teng S., Stainier D.Y., Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset J.C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka S., Ooehara J., Morita Y., Ema H., Takahashi S., Miyawaki A., Otsu M., Yamaguchi T., Onodera M., Nakauchi H. Generation of transgenic mouse line expressing Kusabira Orange throughout body, including erythrocytes, by random segregation of provirus method. Biochem. Biophys. Res. Commun. 2013;435:586–591. doi: 10.1016/j.bbrc.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Hamanaka S., Yamaguchi T., Kobayashi T., Kato-Itoh M., Yamazaki S., Sato H., Umino A., Wakiyama Y., Arai M., Sanbo M. Generation of germline-competent rat induced pluripotent stem cells. PLoS One. 2011;6:e22008. doi: 10.1371/journal.pone.0022008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert S.P., Huisken J., Kim T.N., Feldman M.E., Houseman B.T., Wang R.A., Shokat K.M., Stainier D.Y. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K.K., Majesky M.W. Smooth muscle stem cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;276:22–33. doi: 10.1002/ar.a.10128. [DOI] [PubMed] [Google Scholar]
- Hoeffel G., Wang Y., Greter M., See P., Teo P., Malleret B., Leboeuf M., Low D., Oller G., Almeida F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K., Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Yamaguchi T., Hamanaka S., Kato-Itoh M., Yamazaki Y., Ibata M., Sato H., Lee Y.S., Usui J., Knisely A.S. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Matsunari H., Nagashima H., Watanabe M., Umeyama K., Nakano K., Nagaya M., Kobayashi T., Yamaguchi T., Sumazaki R., Herzenberg L.A. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc. Natl. Acad. Sci. USA. 2013;110:4557–4562. doi: 10.1073/pnas.1222902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Colbert M.C., Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Platt J.L., Fischel R.J., Matas A.J., Reif S.A., Bolman R.M., Bach F.H. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991;52:214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- Platt J.L., Turman M.A., Noreen H.J., Fischel R.J., Bolman R.M., 3rd, Bach F.H. An ELISA assay for xenoreactive natural antibodies. Transplantation. 1990;49:1000–1001. doi: 10.1097/00007890-199005000-00033. [DOI] [PubMed] [Google Scholar]
- Sakurai Y., Ohgimoto K., Kataoka Y., Yoshida N., Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F., Ho J., Stanford W.L., Fischer K.D., Schuh A.C., Schwartz L., Bernstein A., Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L., Schuh A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Usui J., Kobayashi T., Yamaguchi T., Knisely A.S., Nishinakamura R., Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am. J. Pathol. 2012;180:2417–2426. doi: 10.1016/j.ajpath.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Hamanaka S., Kamiya A., Okabe M., Kawarai M., Wakiyama Y., Umino A., Hayama T., Sato H., Lee Y.S. Development of an all-in-one inducible lentiviral vector for gene specific analysis of reprogramming. PLoS One. 2012;7:e41007. doi: 10.1371/journal.pone.0041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Sato H., Kato-Itoh M., Goto T., Hara H., Sanbo M., Mizuno N., Kobayashi T., Yanagida A., Umino A. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542:191–196. doi: 10.1038/nature21070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.