Abstract
Background
Stooped posture was mentioned in the original description of the characteristic features of Parkinson's disease (PD). Since then, a variety of postural, bone, and joint problems have become recognized as common aspects of the illness and deserve attention.
Methods
A Medline literature search for the period from 1970 to 2016 was performed to identify articles relevant to this topic. Keywords for the search included posture, spine, bone disorders, fractures, joint disorders, kyphosis, scoliosis, stooping, camptocormia, Pisa syndrome, frozen shoulder, anterocollis, dropped head syndrome, and pain in combination with PD. The articles were then reviewed to summarize clinical features, frequency, impact, pathophysiology, and treatment options for these conditions.
Results
Postural disorders (kyphoscoliosis, camptocormia, Pisa syndrome, dropped head syndrome), bone mineralization disorders (osteoporosis, bone fractures), and joint disorders (frozen shoulder, dystonia involving joints, joint pain) are often seen in association with PD. Treatment options for these conditions are varied and may include medications, physical therapy, or surgical interventions.
Conclusions
Posture, bone, and joint disorders are common in patients with PD; they often produce added disability, and they may be treatable.
Keywords: bone mineralization, camptocormia, joint disorders, kyphosis, Pisa syndrome, posture disorders
In James Parkinson's 1817 monograph “An Essay on the Shaking Palsy,” which first described the illness that now bears his name, he emphasized that a key clinical feature of Parkinson's disease (PD) is a “propensity to bend the trunk forward.”1 Since then, disturbances of posture, the spine, and other bones and joints have become increasingly recognized as important aspects of the illness. For some patients, these problems are minor additive challenges to the movement problems caused by PD. For others, they dominate the clinical picture and cause substantial disability. We performed a literature search of the Medline database for the period from 1970 to 2016 to systematically review the literature on postural, bone, and joint problems associated with PD. Key search words combined with PD included the following: posture, spine, bone disorders, fractures, joint disorders, kyphosis, scoliosis, stooping, camptocormia, Pisa syndrome, frozen shoulder, anterocollis, dropped head syndrome, and pain. Our review of the available literature is presented below, organized in 3 main sections: disorders of posture (spine), bone disorders, and nonspine joint disorders.
Postural Disorders (Spine)
Kyphoscoliosis
Kyphosis is defined as an over‐curvature or excessive rounding of the spine (Table 1). Scoliosis is a lateral structural curvature, usually coupled with vertebral rotation, that causes an asymmetric deformity of the trunk.2 Despite the emphasis by James Parkinson on forward stooping of posture (kyphosis) in PD, the literature is surprisingly sparse on this topic. More has been written about scoliosis, which is also common and is seen more often in patients with PD than in the general older adult population. Kyphoscoliosis refers to the presence of both scoliosis and kyphosis.
Table 1.
Postural disorders associated with parkinson's disease
| Disorder | Treatment Options |
|---|---|
| Kyphoscoliosis |
|
| Camptocormia |
|
| Pisa syndrome |
|
| Dropped head syndrome |
|
PT, physical therapy; DBS, deep‐brain stimulation surgery; rTMS, repetitive trans‐spinal magnetic stimulation.
Although various genetic, bone metabolic, hormonal, biomechanical, and neuromuscular insults are thought to cause primary idiopathic scoliosis, it has been suggested that, in PD, the deformity might be a motor manifestation of the illness itself.3 In fact, 84% of patients with both parkinsonism and scoliosis exhibit leaning contralateral to the side of early or predominant motor symptoms.4 This suggests that scoliosis may be associated with levels of nigrostriatal dopamine deficiency.
Scoliosis is determined on the basis of the shape of the spine, which appears as an ‘S’ or ‘C’ shape.3 The deformity cannot be improved by passive movement or supine positioning, as is the case with many other postural disorders. In addition to physical disfigurement, kyphotic and scoliotic postures can impair limb use, gait and balance, swallowing, and breathing and also can produce pain and discomfort. Thus, these postural deformities can exacerbate the overall functional disability already present in PD.3
Patients may choose to nonsurgically alleviate associated pain or surgically correct the spinal curvature. Spinal fusion appears to be the only truly corrective solution to date. PD patients are considered to be a particularly high‐risk population for spinal surgery. Ohyama et al. compared 2 different spinal fusion strategies on patients with PD, and although correction was achieved in both groups, those who had instrumentation from the inferior thoracic vertebra to the sacrum experienced numerous complications, including multiple subsequent surgeries, rod breakage, pseudoarthritis, and infection.5 Patients who had instrumentation from the superior thoracic vertebra to the sacrum maintained their postural correction with high satisfaction and minimal complications.5
Spinal braces are generally recommended for adolescents with idiopathic scoliosis to stop curvature progression. A 2015 pilot study investigated the effectiveness of a special brace, the “SpineCor Pain Relief Brace” (Spine Corporation Ltd., Chesterfield, UK), which was designed to reduce pain in adults with degenerative scoliosis.6 Although the participants did not have PD, most reported some pain relief and improvements in functionality and activity, although ease of use remained a concern. Further work is needed to better understand the benefits and limitations of bracing in an older patient population with PD.
Nonsurgical opportunities focus on pain relief rather than spinal positioning. Over‐the‐counter or prescribed analgesics, including nonsteroidal anti‐inflammatory drugs (NSAIDs) like ibuprofen, celecoxib, or acetaminophen, may ease accompanying back pain. Epidural injections can help with pain, while physical therapy may strengthen the back and increase mobility in addition to relieving pain.7
Camptocormia
Camptocormia or “bent spine syndrome” was first described in the medical literature in the 1800s. In 1915, the French neurologists Souques and Rosanoff‐Saloff first proposed the term “camptocormia” from the Greek words “kamptos” (to bend) and “kormos” (trunk).8 Initially, camptocormia was thought to represent a psychogenic conversion reaction. In fact, the term was used during World Wars I and II to describe stressed young soldiers who developed this strange posture, perhaps caused by walking stooped in the battle trenches.9 Camptocormia is a postural disorder characterized by marked flexion of the thoracolumbar spine that worsens during walking, improves while sitting, and disappears in the supine position (Fig. 1).8 Individuals generally can voluntarily straighten their posture while standing, but the spine gradually regains its flexed posture after a few minutes. The abnormal posture can become fixed for some, whereas others have variations due to factors like fatigue, stress, or, in the case of patients with PD, on and off motor fluctuations.9 There are no consensus criteria for diagnosing camptocormia, and although a figure of at least 45 degrees of thoracolumbar flexion while standing or walking that resolves in the supine position has been used,8 others have accepted degrees of flexion as low as 15 degrees.
Figure 1.
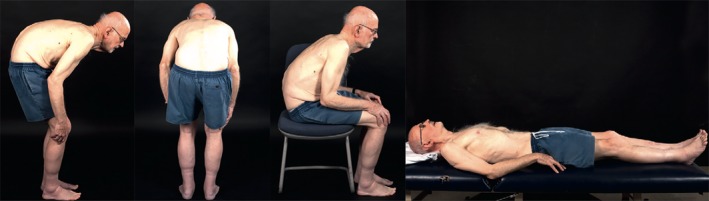
Camptocormia. Trunk flexion is most sever in the standing position and resolves when lying supine. Reprinted from: Doherty et al. ©2011 with permission from Elsevier11.
The exact pathophysiology of camptocormia remains uncertain, but several factors may contribute, including muscular rigidity, axial dystonia, and myopathy of the paraspinal muscles.10 According to Doherty et al., pathological changes in the basal ganglia or affiliated pathways, proprioceptive deficits, loss of postural reflexes, rigidity, and dystonia may contribute to its development.11
Camptocormia is now recognized as a fairly common feature of parkinsonian and dystonic disorders. The reported prevalence of camptocormia in PD varies from 3% to 17.6%, possibly due to the absence of uniform diagnostic criteria and the use of small sample sizes in most studies.3, 12, 13 Camptocormia does not precede other motor features of PD, and patients with camptocormia tend to have more advanced PD than those without it.10, 14 Djaldetti et al. were the first to describe camptocormia in PD in a 1999 case study of 8 patients.9 Development of camptocormia occurred from the time of PD onset to 14 years later. The authors mentioned spondyloarthrotic changes as a potential risk factor for developing camptocormia in PD and suggested that striatal, and especially putaminal, dysfunction could be causative. In addition, the authors proposed that camptocormia might represent a type of axial dystonia or an extreme form of rigidity. Tiple et al. reported no correlation between the degree of camptocormia and clinical or treatment‐related variables, suggesting that nondopaminergic mechanisms were most important.14
Regarding treatment, camptocormia is usually resistant to antiparkinsonian drugs. Levodopa (l‐dopa) is rarely effective and is thought to sometimes induce camptocormia, perhaps as a drug‐induced truncal dystonia. Numerous other treatment options have been tested, including psychotherapy,15 orthotic use in combination with physical therapy,16 intrathecal baclofen,8 corticosteroids,17 protirelin tartrate,18 botulinum toxin,8, 19 immunoglobulins,20 deep‐brain stimulation (DBS),8, 21 and orthopedic surgery,22 but these approaches are not predictably effective.10, 11 The lack of randomized‐controlled trials and the multifactorial etiology of camptocormia complicates treatment for this condition. A 2015 study sought to identify prognostic factors for a beneficial DBS effect on camptocormia and determined that a short duration of camptocormia best predicted improvement from DBS. Patients who had symptoms for no longer than 18 months before neurostimulation experienced the most benefit. Schulz‐Schaeffer et al. mentioned the development of muscle pathology (intrafascicular fibrosis and fatty degeneration) and skeletal degenerative changes as possible reasons why camptocormia could be successfully treated with DBS only within a certain time window.23 In 2014, Arii et al. demonstrated that repetitive trans‐spinal magnetic stimulation could result in a significant short‐term improvement of camptocormia. The mechanism for this effect is not yet known, but by analogy to spinal cord stimulation, afferent blockade of sensory pathways, excitation of descending brainstem motor pathways, or disruption of akinetic corticostriatal activity may be involved. It is a noninvasive intervention that shows promise as a therapy for camptocormia in PD, but careful assessments are needed of the long‐term effects of repetitive trans‐spinal magnetic stimulation therapy on camptocormia. A case report on thoracolumbar spinal fixation for camptocormia in a patient with PD described good sagittal correction, but that patient required subsequent anterior vertebral body fusion and experienced a prolonged and complicated postoperative course. The authors concluded that this option should only be considered for well‐motivated patients who are willing to undergo major reconstructive surgery, and only if DBS is unsuccessful.22
Long‐term complications of camptocormia can include neck and back pain, difficulty swallowing, shortness of breath, visual disruption, degenerative spinal disease, radiculopathies, late‐onset myopathy, soft tissue damage, and dystonia.11 Camptocormia is normally progressive and often becomes a serious impairment to daily life.10
Pisa Syndrome
“Pisa syndrome,” also known as pleurothotonus, refers to a noticeable lateral flexion of the torso and head as well as axial rotation of the torso (Fig. 2). This abnormal tilted posture is reminiscent of the leaning tower of Pisa, hence its name.3 Symptoms typically intensify in standing and sitting positions and resolve in the supine position. Although there are no formal diagnostic criteria for Pisa syndrome, Doherty et al. have suggested that a diagnosis of Pisa syndrome may be made if lateral flexion of the trunk is ≥10 degrees when standing and if passive movement or supine positioning can resolve the abnormal posture.11 Pisa syndrome may develop acutely over several months or gradually over several years. A propensity to lean to 1 side when sitting and mild lateral flexion when walking are often the first symptoms observed in Pisa syndrome. As the condition progresses, patients may begin to experience pain, gait imbalance, frequent falls, and dyspnea.11
Figure 2.

A patient with Pisa syndrome exhibiting substantial lateral flexion to the right side. Adapted from: Tassorelli et al. ©2012 with permission from Wiley.28
In 1972, Ekbom et al. first described Pisa syndrome as an acute axial dystonia triggered by prolonged exposure to neuroleptic drugs.24 Although Pisa syndrome is still linked to the use of antipsychotics, it can also occur as a side effect of other medications like cholinesterase inhibitors, and it also is viewed as a possible comorbidity of neurodegenerative diseases like PD.25 It has been suggested that cholinesterase inhibitors, when used to treat dementia, may induce Pisa syndrome in patients with PD, because it has been proposed that the primary neurochemical pathology in Pisa syndrome might be cholinergic excess or perhaps excess activity of cholinergic neurotransmission relative to dopaminergic, as occurs in PD.3 In support of cholinergic hypotheses, it has been reported that about 40% of patients with Pisa syndrome respond to anticholinergic medication therapy.3 Other neurotransmitters, including serotonin and norepinephrine, have also been implicated in the pathogenesis of Pisa syndrome.24
Castrioto et al. reviewed the possible pathophysiological mechanisms for Pisa syndrome and concluded that asymmetrical basal ganglia functioning was the most important contributor. This is thought to cause asymmetric regulation of postural muscle tone and altered integration of sensory information, resulting in a misperception of body orientation and the adoption of an asymmetric posture.26 Vaugoyeau and Azulay suggested that patients with PD have impaired body orientation and that they might be deficient in integrating visual, vestibular, and proprioceptive input, which, in turn, can induce postural bending.27 In contrast to this, Cannas et al. proposed that asymmetric muscular tone in paraspinal or trunk muscles, exacerbated by the rigidity of PD, might induce lateral trunk flexion; asymmetrical ipsilateral or contralateral hypertrophy of the paraspinal muscles can often be visually detected in patients with Pisa syndrome.24
Most patients who have PD with Pisa syndrome tilt toward the less affected side of the body, whereas approximately 34% tilt to the more affected side.26 Patients who have PD with Pisa syndrome sometimes experience more severe motor asymmetry than those without it.28 A recent Italian multicenter study of Pisa syndrome in patients with PD found the condition present in 8.8% of 1631 patients. Those with the Pisa syndrome were older and had longer disease duration, more advanced illness, more frequent falls, and a poorer quality of life.29 They also received higher doses of l‐dopa and were more likely to be treated with both l‐dopa and a dopamine agonist. Osteoporosis and arthrosis were more common in those with Pisa syndrome.
The management of Pisa syndrome remains a challenge. In some cases, discontinuation of cholinesterase inhibitors, if prescribed, or administration of an anticholinergic restored normal posture.26 Botulinum toxin injections have also been mentioned as a possible treatment.30, 31 Over time, Pisa syndrome may induce secondary changes in muscles, connective tissues, and bone, resulting in scoliosis.24
Dropped Head Syndrome
Dropped head syndrome, also known as forward neck flexion or anterocollis, is considered a rare feature of PD. The term “disproportionate antecollis” has also been used, mainly to signify that the anterocollis is exaggerated compared with truncal postural changes, but this term has typically been applied to discussions of dropped head syndrome in patients with multiple system atrophy. Although incidence estimates in PD are generally around 6%, dropped head syndrome can severely disturb the quality of life in affected patients.32, 33
Dropped head is characterized by a chin‐on‐chest deformity due to neck flexion, sometimes with minor thoracic or lumbar spinal curvature, that may be correctable by passive neck extension (Fig. 3).11, 33 Specifically, anterocollis is defined as marked neck flexion of 45 degrees or more that is accompanied by an inability to lift the head in sitting or standing positions. The neck flexion is out of proportion to truncal flexion but may be voluntarily overcome in early stages.11, 33 A patient with dropped head syndrome is sometimes able to transiently lift the head out of the flexed position, although the abnormal posture soon returns.3 Protruding neck extensor muscles that are firm on palpation is an additional useful sign. In patients with PD, the deformity often develops over the course of several days and is exacerbated during off periods. Kashihara et al. reported that the mean illness duration in patients with PD who developed anterocollis was 5.4 ± 4.3 years, with the onset of dropped head occurring from 6 months before initial motor symptoms to 15 years afterward.34
Figure 3.

Dropped head is characterized by a chin‐on‐chest deformity due to neck flexion, sometimes with minor thoracic or lumbar spinal curvature, that may be correctable by passive neck extension. Reprinted from: Kashihara et al. ©2006 with permission from Wiley.34
A complete inability to normalize neck flexion may be seen with the progression of dropped head syndrome. This may result in muscle contracture, secondary spinal degenerative changes, and cervical myelopathy.35 Patients report that pain may accompany dropped head syndrome as a result of involuntary muscle spasm or secondary or associated degenerative joint disease of the cervical spine.3 It has been reported that the positioning sometimes restores itself to the upright or extended position in the later stages of PD, perhaps because tonus of the neck extensor muscles may come to exceed that of the flexor muscles.24 In addition, anterocollis may recur as PD progresses, even in patients who respond well to initial treatment.
Although the pathogenesis is debated, most believe that dropped head is caused by dystonic muscle contractions (anterocollis) or a myopathy of neck extensor muscles that results in a dropped head position.3 It is also thought that increased axial rigidity may underlie the condition in PD.24 Risk factors for dropped head syndrome include female gender, PD severity, degree of rigidity, and the akinesia‐dominant PD subtypes. Patients who have PD with a tremor‐dominant subtype usually do not develop dropped head syndrome. For some, dopaminergic medications, especially dopamine agonists, can improve dropped head. For others, these drugs are not helpful or can even worsen the problem, perhaps by inducing dystonic dyskinesias.24 In patients whose dropped head appeared after introducing or increasing dopaminergic drugs, symptoms may improve after drug reduction or discontinuation. In a study by Kashihara et al., 8 of 15 patients who had PD with dropped head syndrome showed improvement after adjustments of their dopaminergic medication.34
The best treatment for dropped head syndrome is unclear. “Sensory tricks,” which are often used in primary cervical dystonia, are usually ineffective when applied to dropped head.24 To date, muscle afferent blocking using lidocaine and ethanol or botulinum toxin seems most effective. Muscle afferent blocking, if performed correctly, can reduce muscle activity, easing chin‐to‐chest positioning without causing weakness of the muscles.36 Botulinum toxin may also reduce pain and muscle stiffness associated with anterocollis but is considered less effective than lidocaine in treating the postural abnormality component.36 It has been reported that DBS can partially improve dropped head, but there is not enough evidence to justify the use of this technique solely to treat this problem, because DBS has not been found to have demonstrable benefit in other reports.32, 36 Physical therapy may be helpful, but its efficacy needs further exploration. Wearing a soft neck brace to elevate the head may improve the patient's vision and reduce physical and mental discomfort.34
Cervical dystonia aside from the dropped head presentation may also occur in patients who have PD as a dystonic component of the illness or as a manifestation of drug‐induced dystonic dyskinesias.37, 38
Bone Mineralization
Osteoporosis
Osteoporosis is a skeletal disorder characterized by decreased bone mass and strength due to various factors that include increased bone turnover, reduced bone mineralization, and deteriorating bone architecture (Table 2). The World Health Organization defines osteoporosis on the basis of bone densitometry scans as a bone mineral density (BMD) less than 2.5 standard deviations below the average for age, race, and gender.39 Patients are considered osteopenic if the BMD is between 1.0 and 2.5 standard deviations below the mean. There are multiple risk factors for osteoporosis, and the most significant of these are older age, female gender, reduced physical activity, and vitamin D deficiency. It is now recognized that the risk for osteoporosis is significantly increased in patients with PD. Studies have demonstrated that patients with PD have a BMD between 4.8% and 6.6% lower than controls adjusting for site of measurement, age, and gender.40, 41, 42, 43 Other studies have confirmed a significant decrease in BMD in patients who have PD compared with healthy individuals.44 Major associated factors in patients with PD are immobility, decreased body mass index, and vitamin D deficiency.45
Table 2.
Bone mineralization disorders associated with parkinson's disease
| Disorder | Treatment Options |
|---|---|
| Osteoporosis |
|
| Bone fractures | Prevention through:
|
PT, physical therapy.
Much of the research on osteoporosis has focused on vitamin D deficiency as a major cause. There are multiple forms of vitamin D, and synthesis of the active form, 1,25‐dihydroxyvitamin D (1,25‐[OH]2D), begins when ultraviolet light is absorbed through the skin, converting a form of stored cholesterol into vitamin D3. Through other mechanisms, the liver and kidneys convert vitamin D3 into 1,25‐(OH)2D, which is then transported to the bones to increase mineralization and strength. Based on the understanding of vitamin D metabolism, it has been concluded that patients with PD tend to have an increased risk for vitamin D deficiency due to reduced sunlight exposure, poor dietary intake, and decreased physical activity.46
Bone Fractures
It has been demonstrated that patients with PD aged 65 years and older have a higher incidence of fractures compared with the general elderly population.47 One population study demonstrated that patients with PD had a new fracture rate of 46% within 10 years versus 29% in control groups, with females having a significantly higher risk than males.48 In PD, fractures are most commonly located in the femur.47 Increased fracture risk in PD may be attributed to postural instability with difficulty in transfers and increased falls, orthostatic hypotension, and decreased BMD. Anticholinergic drugs used to treat PD are also associated with a higher risk independent of the stage of the disease, probably due to the negative effects of anticholinergics on postural balance and cognition. Similarly, patients with PD who are being treated for depression with tricyclic antidepressants have also been shown to be at increased risk of fractures due to the medications’ anticholinergic effects.49 After accounting for confounders, such as history of falls, age, and gender, the significantly decreased BMD has also been positively correlated with an increased number of fractures.47 The risk of fracture is significantly higher in women with PD, because they are more likely to have a reduced BMD, especially after menopause.47
Treatment for osteoporosis and prevention of fractures in patients with PD focuses on addressing the major associated factors. Sato et al. (1999) demonstrated in a randomized, double‐blind, placebo‐controlled trial of 86 patients with PD that supplementation with 1α‐hydroxyvitamin D3 in those who had low serum 1,25‐(OH)2D levels significantly reduced the risk for fracture.50 Those authors showed that, although there was a continued reduction in BMD in both the treatment and nontreatment groups over the course of 18 months, the decline was accelerated in the untreated group. The fall frequency was equal in both groups, so it was concluded that the decreased fracture rate was caused by the relatively higher BMD in the vitamin D‐supplemented group compared with the placebo group. Other treatments to consider for increasing BMD are hormone‐replacement therapy for female patients and nutritional support to improve body mass index.45 Suzuki et al. recently reported that, in a randomized, double‐blind, placebo‐controlled trial, supplementation with vitamin D3 significantly delayed progression of PD.51 This was thought to be related to the high concentration of vitamin D receptors on nigral dopaminergic neurons. Thus, vitamin D may have a wider role in the treatment of PD beyond its actions in bone metabolism.43
Attention to bone issues should also address contributors to falling. Optimizing medication therapy for PD to minimize postural instability, physical therapy, the use of assistive gait devices, and appropriate treatment of orthostatic hypotension and perhaps cognition may help to reduce falls. It has been demonstrated that resistive exercise not only increases BMD but also reduces falls.52 Tai chi also reportedly improves postural stability and mitigates falling.53
Joint Problems
Frozen Shoulder
Frozen shoulder, also called periarthritis or adhesive capsulitis, is a condition in which there is spontaneous onset of pain and gradual restriction of range of motion (ROM) in a shoulder in the absence of any discernible joint abnormality (Table 3).54 Frozen shoulder can appear within 1 or 2 years before the onset of typical motor features in patients with PD and can be considered a “premotor” feature of the illness.54 The characteristic features of frozen shoulder include slow onset of pain, pain at the insertion of the deltoid, sleeplessness, painful and limited elevation and external rotation with restriction of both the active and passive types, atrophy of the infraspinatus, and minor localized tenderness. The course of frozen shoulder is described as having 3 distinct chronological stages: a freezing/painful stage that lasts from 3 to 9 months, a frozen/transitional stage that lasts from 4 to 12 months and is characterized by waning pain and highly limited ROM (during this stage, external shoulder rotation is most restricted, followed closely by shoulder flexion and internal rotation), and a thawing stage that lasts from 12 to 42 months and starts when ROM begins to improve and ends once ROM fully returns.55 Frozen shoulder rarely occurs in both shoulders simultaneously, but it may occur sequentially and bilaterally in 40% to 50% of patients.55 Frozen shoulder can clinically resemble other shoulder conditions early on, including major trauma, rotator cuff tear or contusion, and subacromial bursitis. The diagnosis is often 1 of exclusion. If no other shoulder pathologies are identified and if radiographs do not reveal osteoarthritis or other abnormalities, then the diagnosis of frozen shoulder can be given.55 The pathophysiological process is not completely known but is believed to involve synovial inflammation and fibrosis of the joint capsule.
Table 3.
Joint disorders associated with parkinson's disease
| Disorder | Treatment Options |
|---|---|
| Frozen shoulder |
|
| Dystonia across joints |
|
| Joint pain |
|
PT, physical therapy; DBS, deep‐brain stimulation surgery.
Aside from the 1989 publication by Riley et al.,54 there is sparse information about the incidence of frozen shoulder in patients with PD, although it is frequently encountered in clinical practice. Riley et al. suggested that the occurrence of frozen shoulder in their PD patients was secondary to the akinesia of PD rather than being a primary or causative event in itself. They reported that the peak occurrence of frozen shoulder was during the 2 years before onset of the usual PD symptoms and that, in almost all cases, the initial symptoms of PD developed in the upper limb ipsilateral to the frozen shoulder. Among patients who had PD with frozen shoulder, akinesia was twice as likely as tremor to be the first PD symptom, whereas the ratio was nearly reversed for patients who had PD without frozen shoulder.54 The authors proposed the addition of PD to the list of predisposing causes for frozen shoulder, with the mechanism involving lack of movement about the joint brought on by akinesia.54
Numerous nonsurgical and surgical treatment options exist for frozen shoulder. Physical rehabilitation has been shown to minimize pain, increase ROM, and restore function.56 NSAIDs or corticosteroids may be used during any stage in an attempt to relieve symptoms.55 Studies indicate that use of oral corticosteroids may be effective in relieving pain in the short term, and there is some evidence to suggest that intra‐articular corticosteroid injections also provide a short‐term benefit.57, 58 Capsular distension involves the injection of a local anesthetic into the joint in an attempt to stretch the capsule. Although helpful, this technique may be poorly tolerated because of pain during the procedure.55, 59
Surgical treatment can be considered if nonsurgical measures fail. Manipulation under anesthesia should allow ROM to return in the operating room, and there is often continued relief with postoperative physical therapy. Arthroscopic release and repair has become a widely accepted treatment for frozen shoulder. An advantage of arthroscopy is that it allows for a complete assessment of shoulder anatomy, and any undiagnosed abnormalities that may have contributed to frozen shoulder development can be addressed. On average, after surgical intervention, it takes about 2.8 months for the shoulder to be pain free and regain full ROM.55
Effects on Joints from Dystonia
Dystonia is characterized by persistent muscle contraction associated with repetitive twisting movements, abnormal postures, or both. Focal dystonia—dystonia affecting only 1 body part—is commonly encountered in PD and is often seen in limbs and across joints, especially the hands and feet. Dystonia can cause joint area pain and immobility, adding to the movement problems of PD. Dystonia involving the trunk, hip, and legs can lead to peculiar gaits in patients with PD. Jankovic and Tintner determined that, in the clinical setting, dystonia was diagnosed in at least 30% of patients with PD and in up to 60% of patients who experienced the onset of PD before 40 years of age.37 The side of dystonic deformity is usually the side of initial PD signs. Ashour and Jankovic reported that this was the case in 93.1% of their patients with striatal hand or foot dystonia and suggested that clinicians search for dystonia as evidence for the side of initial PD presentation.3 In addition, feet are only rarely involved in adult primary dystonia; thus, when dystonia affects the foot in an adult, the possibility of PD should be explored.37
Purves Stewart, who described movement‐induced foot dystonia in his PD patients during the late 1800s, was the first to describe dystonia as both an initial and late feature of PD. Dystonia in PD is now understood to be either a comorbidity or a drug‐induced phenomenon from dopaminergic treatment.60 Their close association could imply a shared pathophysiology for dystonia and bradykinesia, rigidity, and tremor, but dystonia sometimes becomes less pronounced or even resolves despite progressive parkinsonism. In addition, antiparkinsonian drugs influence dystonic spasms with high inconsistency; l‐dopa–induced dystonia is seen as an off‐period, biphasic, or peak‐dose phenomenon. Off‐period dystonia is the most common type of drug‐induced dystonia in PD, with an overall occurrence varying from 20% to 33% of patients who receive treatment for longer than 3 years.60 Off‐period dystonia is also more likely to result in pain compared with biphasic or peak‐dose dystonia and most commonly involves the feet, usually ipsilateral to the side most affected by PD.37, 60 Peak‐dose dystonia generally affects the orofacial region, platysma, and neck.60
Aside from adjusting dopaminergic agents, other therapeutic strategies include oral medications (baclofen, anticholinergics, and benzodiazepines), local injections of botulinum toxin, intrathecal baclofen, surgical lesions, tendon transfers and releases, or high‐frequency DBS.37
Joint Pain
Patients with PD may experience a variety of painful sensations, often localized to joints. Such pain is often accentuated by aspects of PD itself, such as prolonged and abnormal postures, disturbed mechanics of gait, falls, immobility, inactivity, or drug‐induced dyskinesias, either choreiform or dystonic forms.61
Joint pain commonly occurs in PD, most frequently in the shoulder, hips, knees, and ankles.61 One case‐control study reported that, after adjustment for age, gender, and previous shoulder injury, patients with PD were 21 times more likely to experience shoulder pain, highlighting the prevalence of this issue in the PD population.62 Joint pain tends to increase during times of increased immobility, such as during off periods, and females with PD report neck and back pain more often than males.61, 63 Virtually any type of joint disorder can be encountered in patients with PD, including osteoarthritis, rheumatoid arthritis, other connective tissue disorders, traumatic arthritis, Charcot joints,64 infectious arthritis, plantar fasciitis, temporomandibular joint disorder, bursitis, and others. Little information is available regarding the relative incidence of these conditions in PD compared with the general population. Prolonged flexion of the wrist due to lack of normal arm movements and body repositioning during sleep or because of dystonic posturing at this joint can lead to median nerve compression (carpal tunnel syndrome). Spinal arthritis may give rise to painful radiculopathies. Sometimes the pain, paresthesias, and dysesthesias caused by neuropathies or radiculopathies may be difficult to distinguish from nonmotor sensory symptoms of PD.
There is little published information about the optimum treatment of joint pain in patients with PD. If motor symptoms induce or exacerbate joint pain, then adjustments to dopaminergic medications can provide some relief.65 If joint pain persists, then physical therapy can improve mobility and also provide pain relief.65 Traditional medications, including NSAIDs, cyclooxygenase‐2 inhibitors, and analgesics, and drugs for chronic pain, such as amitriptyline, gabapentin, and pregabalin, may also be employed.66 Other treatment modalities include intra‐articular steroid injections or intra‐articular surgical procedures, which also ease joint pain.66 In patients with refractory pain, joint replacement remains an option. Although surgical complications are more prevalent in this population, perioperative mortality rates remain comparable to those of non‐PD controls.67 When indicated, hip replacements in particular have been found to provide short‐term functional improvement as well as significant pain relief.68
Conclusion
Postural, bone, and joint disorders remain a source of impairment for many patients with PD. Future therapeutic research that addresses these conditions in the context of PD is needed, because treatment options are often limited. Optimal care of patients with PD should include attention to these disorders, because they often contribute to disability.
Author Roles: 1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.L.R.: 1A, 1B, 1C, 3A, 3B
M.C.E.: 1B, 1C, 3A
A.P.: 1B, 1C, 3A
I.G.: 1C, 3B
R.K.: 1A, 1B, 1C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: Marcie L. Rabin reports personal fees from Teva and Allergan and research support from Ipsen and Osmotica. Roger Kurlan reports personal fees from Teva and research support from Astra‐Zeneca, Neurocrine, Synchroneuron, Phytopharm, and the National Institutes of Health.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Pearce JM. Aspects of the history of Parkinson's disease. J Neurol Neurosurg Psychiatry 1989;(Suppl):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baik JS, Kim JY, Park JH, Han SW, Park JH, Lee MS. Scoliosis in patients with Parkinson's disease. J Clin Neurol 2009;5:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord 2006;21:1856–1863. [DOI] [PubMed] [Google Scholar]
- 4. Duvoisin RC, Marsden CD. Note on the scoliosis of Parkinsonism. J Neurol Neurosurg Psychiatry 1975;38:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohyama Y, Iida T, Katayanagi J, et al. Range of instrumentation and complications in the surgical treatment of spinal deformity in patients with Parkinson's disease [abstract]. Scoliosis 2015;10(suppl 1):P26. [Google Scholar]
- 6. Park A, Bettany‐Saltikov J, Cole A, Ling J. An investigation into the effects of the SpineCor Pain Relief Brace on adults with degenerative scoliosis [abstract]. Bone Joint J 2015;97(suppl 2):4. [Google Scholar]
- 7. Nam HS, Park YB. Effects of transforaminal injection for degenerative lumbar scoliosis combined with spinal stenosis. Ann Rehabil Med 2011;35:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology 2005;65:355–359. [DOI] [PubMed] [Google Scholar]
- 9. Djaldetti R, Mosberg‐Galili R, Sroka H, Merims D, Melamed E. Camptocormia (bent spine) in patients with Parkinson's disease—characterization and possible pathogenesis of an unusual phenomenon. Mov Disord 1999;14:443–447. [DOI] [PubMed] [Google Scholar]
- 10. Arii Y, Sawada Y, Kawamura K, et al. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2014;85:1221–1226. [DOI] [PubMed] [Google Scholar]
- 11. Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson's disease. Lancet Neurol 2011;10:538–549. [DOI] [PubMed] [Google Scholar]
- 12. Lepoutre AC, Devos D, Blanchard‐Dauphin A, et al. A specific clinical pattern of camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abe K, Uchida Y, Notani M. Camptocormia in Parkinson's disease [serial online]. Parkinsons Dis 2010;2010:pii267640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiple D, Fabbrini G, Colosimo C, et al. Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry 2009;80:145–148. [DOI] [PubMed] [Google Scholar]
- 15. Skidmore F, Anderson K, Fram D, Weiner W. Psychogenic camptocormia. Mov Disord 2007;22:1974–1975. [DOI] [PubMed] [Google Scholar]
- 16. de Seze MP, Creuze A, de Seze M, Mazaux JM. An orthosis and physiotherapy programme for camptocormia: a prospective case study. J Rehabil Med 2008;40:761–765. [DOI] [PubMed] [Google Scholar]
- 17. Hilliquin P, Menkes CJ, Laoussadi S, Job‐Deslandre C, Serratrice G. Camptocormia in the elderly. A new entity by paravertebral muscle involvement [article in French]? Rev Rhum Mal Osteoartic 1992;59:169–175. [PubMed] [Google Scholar]
- 18. Takei A, Hamada S, Homma S, Hamada K, Tashiro K, Hamada T. Amelioration of subacute camptocormia in multiple system atrophy by protirelin tartrate. Mov Disord 2009;24:2022–2023. [DOI] [PubMed] [Google Scholar]
- 19. von Coelln R, Raible A, Gasser T, Asmus F. Ultrasound‐guided injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord 2008;23:889–892. [DOI] [PubMed] [Google Scholar]
- 20. Dominick J, Sheean G, Schleimer J, Wixom C. Response of the dropped head/bent spine syndrome to treatment with intravenous immunoglobulin. Muscle Nerve 2006;33:824–826. [DOI] [PubMed] [Google Scholar]
- 21. Sako W, Nishio M, Maruo T, et al. Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson's disease. Mov Disord 2009;24:1076–1079. [DOI] [PubMed] [Google Scholar]
- 22. Peek AC, Quinn N, Casey AT, Etherington G. Thoracolumbar spinal fixation for camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2009;80:1275–1278. [DOI] [PubMed] [Google Scholar]
- 23. Schulz‐Schaeffer WJ, Margraf NG, Munser S, et al. Effect of neurostimulation on camptocormia in Parkinson's disease depends on symptom duration. Mov Disord 2015;30:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kashihara K. Postural disorders in Parkinson's disease: clinical characteristics, frequency, pathophysiology and management. Neurodegener Dis Manag 2012;2:577–588. [Google Scholar]
- 25. Villarejo A, Camacho A, Garcia‐Ramos R, et al. Cholinergic‐dopaminergic imbalance in Pisa syndrome. Clin Neuropharmacol 2003;26:119–121. [DOI] [PubMed] [Google Scholar]
- 26. Castrioto A, Piscicelli C, Perennou D, Krack P, Debu B. The pathogenesis of Pisa syndrome in Parkinson's disease. Mov Disord 2014;29:1100–1107. [DOI] [PubMed] [Google Scholar]
- 27. Vaugoyeau M, Azulay JP. Role of sensory information in the control of postural orientation in Parkinson's disease. J Neurol Sci 2010;289(1–2):66–68. [DOI] [PubMed] [Google Scholar]
- 28. Tassorelli C, Furnari A, Buscone S, et al. Pisa syndrome in Parkinson's disease: clinical, electromyographic, and radiological characterization. Mov Disord 2012;27:227–235. [DOI] [PubMed] [Google Scholar]
- 29. Tinazzi M, Fasano A, Geroin C, et al. Pisa syndrome in Parkinson disease: an observational multicenter Italian study. Neurology 2015;85:1769–1779. [DOI] [PubMed] [Google Scholar]
- 30. Dupeyron A, Viollet E, Coroian F, Gagnard C, Renard D, Castelnovo G. Botulinum toxin‐A for treatment of Pisa syndrome: a new target muscle. Parkinsonism Relat Disord 2015;21:669–670. [DOI] [PubMed] [Google Scholar]
- 31. Tassorelli C, De Icco R, Alfonsi E, et al. Botulinum toxin type A potentiates the effect of neuromotor rehabilitation of Pisa syndrome in Parkinson disease: a placebo controlled study. Parkinsonism Relat Disord 2014;20:1140–1144. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto K. Dropped head in Parkinson's disease. J Neurol 2006;253(suppl 7):VII21–VII26. [DOI] [PubMed] [Google Scholar]
- 33. Revuelta GJ. Anterocollis and camptocormia in parkinsonism: a current assessment. Curr Neurol Neurosci Rep 2012;12:386–391. [DOI] [PubMed] [Google Scholar]
- 34. Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson's disease. Mov Disord 2006;21:1213–1216. [DOI] [PubMed] [Google Scholar]
- 35. Martin AR, Reddy R, Fehlings MG. Dropped head syndrome: diagnosis and management. Evid Based Spine Care J 2011;2:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oyama G, Hayashi A, Mizuno Y, Hattori N. Mechanism and treatment of dropped head syndrome associated with parkinsonism. Parkinsonism Relat Disord 2009;15:181–186. [DOI] [PubMed] [Google Scholar]
- 37. Jankovic J, Tintner R. Dystonia and parkinsonism. Parkinsonism Relat Disord 2001;8:109–121. [DOI] [PubMed] [Google Scholar]
- 38. Tolosa E, Compta Y. Dystonia in Parkinson's disease. J Neurol 2006;253(suppl 7):VII7–VII13. [DOI] [PubMed] [Google Scholar]
- 39. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994;4:368–381. [DOI] [PubMed] [Google Scholar]
- 40. Schneider JL, Fink HA, Ewing SK, Ensrud KE. Cummings SR; Study of Osteoporotic Fractures (SOF) Research Group. The association of Parkinson's disease with bone mineral density and fracture in older women. Osteoporos Int 2008;19:1093–1097. [DOI] [PubMed] [Google Scholar]
- 41. Fink HA, Kuskowski MA, Orwoll ES, Cauley JA, Ensrud KE. Association between Parkinson's disease and low bone density and falls in older men: the osteoporotic fractures in men study. J Am Geriatr Soc 2005;53:1559–1564. [DOI] [PubMed] [Google Scholar]
- 42. Taggart H, Crawford V. Reduced bone density of the hip in elderly patients with Parkinson's disease. Age Ageing 1995;24:326–328. [DOI] [PubMed] [Google Scholar]
- 43. Kao CH, Chen CC, Wang SJ, Chia LG, Yeh SH. Bone mineral density in patients with Parkinson's disease measured by dual photon absorptiometry. Nucl Med Commun 1994;15:173–177. [DOI] [PubMed] [Google Scholar]
- 44. Torsney KM, Noyce AJ, Doherty KM, Bestwick JP, Dobson R, Lees AJ. Bone health in Parkinson's disease: a systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry 2014;85:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vaserman N. Parkinson's disease and osteoporosis. Joint Bone Spine 2005;72:484–488. [DOI] [PubMed] [Google Scholar]
- 46. Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol 2008;65:1348–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato Y, Kaji M, Tsuru T, Oizumi K. Risk factors for hip fracture among elderly patients with Parkinson's disease. J Neurol Sci 2001;182:89–93. [DOI] [PubMed] [Google Scholar]
- 48. Melton LJ 3rd, Leibson CL, Achenbach SJ, et al. Fracture risk after the diagnosis of Parkinson's disease: influence of concomitant dementia. Mov Disord 2006;21:1361–1367. [DOI] [PubMed] [Google Scholar]
- 49. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with parkinsonism and anti‐Parkinson drugs. Calcif Tissue Int 2007;81:153–161. [DOI] [PubMed] [Google Scholar]
- 50. Sato Y, Manabe S, Kuno H, Oizumi K. Amelioration of osteopenia and hypovitaminosis D by 1alpha‐hydroxyvitamin D3 in elderly patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 1999;66:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suzuki M, Yoshioka M, Hashimoto M, et al. Randomized, double‐blind, placebo‐controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr 2013;97:1004–1013. [DOI] [PubMed] [Google Scholar]
- 52. Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord 2008;23:1–11. [DOI] [PubMed] [Google Scholar]
- 53. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med 2012;366:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Riley D, Lang AE, Blair RD, Birnbaum A, Reid B. Frozen shoulder and other shoulder disturbances in Parkinson's disease. J Neurol Neurosurg Psychiatry 1989;52:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manske RC, Prohaska D. Diagnosis and management of adhesive capsulitis. Curr Rev Musculoskelet Med 2008;1(3–4):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Griggs SM, Ahn A, Green A. Idiopathic adhesive capsulitis. A prospective functional outcome study of nonoperative treatment. . J Bone Joint Surg Am 2000;82‐A:1398–1407. [PubMed] [Google Scholar]
- 57. Buchbinder R, Hoving JL, Green S, Hall S, Forbes A, Nash P. Short course prednisolone for adhesive capsulitis (frozen shoulder or stiff painful shoulder): a randomised, double blind, placebo controlled trial. Ann Rheum Dis 2004;63:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carette S, Moffet H, Tardif J, et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo‐controlled trial. Arthritis Rheum 2003;48:829–838. [DOI] [PubMed] [Google Scholar]
- 59. Park KD, Nam HS, Lee JK, Kim YJ, Park Y. Treatment effects of ultrasound‐guided capsular distension with hyaluronic acid in adhesive capsulitis of the shoulder. Arch Phys Med Rehabil 2013;94:264–270. [DOI] [PubMed] [Google Scholar]
- 60. Poewe WH, Lees AJ, Stern GM. Dystonia in Parkinson's disease: clinical and pharmacological features. Ann Neurol 1988;23:73–78. [DOI] [PubMed] [Google Scholar]
- 61. Goetz CG, Tanner CM, Levy M, Wilson RS, Garron DC. Pain in Parkinson's disease. Mov Disord 1986;1:45–49. [DOI] [PubMed] [Google Scholar]
- 62. Madden MB, Hall DA. Shoulder pain in Parkinson's disease: a case‐control study. Mov Disord 2010;25:1105–1106. [DOI] [PubMed] [Google Scholar]
- 63. Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson's disease symptom profile. Acta Neurol Scand 2000;102:37–43. [DOI] [PubMed] [Google Scholar]
- 64. Loriaut P, Rozenberg S, Boyer P, et al. Charcot spine and Parkinson's disease [serial online]. Case Rep Orthop 2014;2014:631346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ford B. Pain in Parkinson's disease. Mov Disord 2010;25(suppl 1):S98–S103. [DOI] [PubMed] [Google Scholar]
- 66. Altman RD, Hochberg MC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000;43:1905–1915. [DOI] [PubMed] [Google Scholar]
- 67. Jamsen E, Puolakka T, Peltola M, Eskelinen A, Lehto MU. Surgical outcomes of primary hip and knee replacements in patients with Parkinson's disease: a nationwide registry‐based case‐controlled study. Bone Joint J 2014;96‐B:486–491. [DOI] [PubMed] [Google Scholar]
- 68. Weber M, Cabanela ME, Sim FH, Frassica FJ, Harmsen WS. Total hip replacement in patients with Parkinson's disease. Int Orthop 2002;26:66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


