We report a patient with Huntington's disease (HD) who presented with repetitive, violent nocturnal movements. The case represents a novel sleep‐related movement disorder in HD and underscores the importance of identification of nocturnal movements and their relationship to sleep.
Patient and Methods
A 52‐year‐old man with HD presented with 10 years of episodes of repetitive, quick leg movements that consisted of ab‐ and adduction of the legs, causing severe bruising of the ankles. Episodes started within the first hour of sleep and recurred in waves through the first half of the night, sparing the day. He was unresponsive during episodes. His wife reported snoring. Melatonin, trazodone, and temazepam for presumed rapid eye movement (REM) behavior disorder were ineffective.
HD was diagnosed 2 years before with symptoms of clumsiness, irritability, and poor concentration. He had 43 cytosine‐adenine‐guanine (CAG) repeats.
Results
He was admitted to the epilepsy monitoring unit. On exam, he had mild chorea affecting distal upper extremities, not involving legs, normal attention and cognition, but poor insight. Video EEG captured two types of episodes. Both emerged after arousals during non‐REM (NREM) sleep. The first was limited to brief, apneic arousals with snoring and consisted of an apneic “jerk” and emergence of generalized chorea that lasted for 10 to 30 seconds before returning to NREM sleep (Fig. 1A; see Video 1, type A). The second occurred after more sustained awakenings with repetitive ballistic movement (RBM) consisting of quasi‐rhythmic (0.5–2.0 Hz) violent movement of legs (Fig. 1B; see Video 1, type B). No other sleep‐related movements and no epileptiform discharges were present. An arousal index of 70 arousals per hour was calculated during a 1‐hour sample, most associated with probable apneas. No evidence of REM movements or absence of atonia occurred; in fact, only a few epochs of REM or deep sleep were evident, with most sleep consisting of N1/N2.
Figure 1.
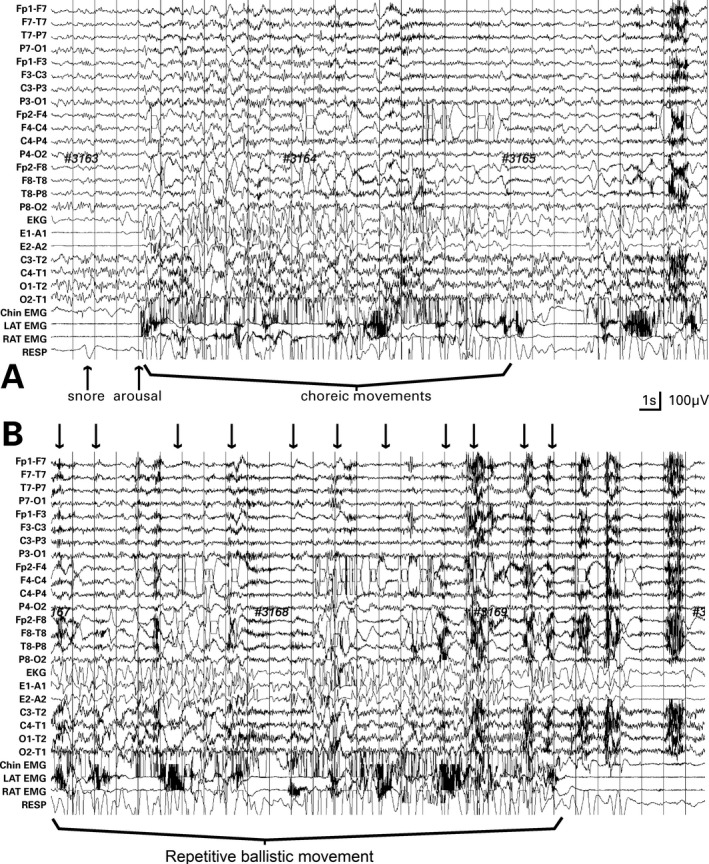
Two 30‐second samples of video EEG; standard 10 to 20 electrode placement. (A) Post‐arousal chorea during NREM sleep: an apneic arousal (arrow) leads to cessation of apnea and emergence of chorea. (B) Twenty seconds after sample (A), repetitive ballistic movement of legs appear (arrows)
Formal polysomnography (PSG) performed 6 weeks postdischarge showed that RBM did not occur during REM and primarily occurred in arousal after events in NREM sleep. A follow‐up visit 5 months later revealed that treatment with continuous positive airway pressure improved nocturnal movements.
Discussion
We found two nocturnal movements associated with arousals or awakenings: the first typical chorea, the second RBM. The case is of interest for the following reasons. First, RBM has not been previously described as a sleep‐related movement disorder in HD. Second, both chorea and RBM occurred during the waking state subsequent to arousals and attenuated with sleep similar to other movement disorders, such as tremor. Third, diagnosis of nocturnal movements and the cause of fragmented sleep required overnight video EEG monitoring for characterization, leading to the recognition that a typical sleep disorder—probable obstructive sleep apnea—triggered awakenings and abnormal movements.
Although motor and cognitive symptoms of HD are well described, its sleep disorders are less so. Animal models of HD demonstrate fragmented sleep with circadian disorganization (see Morton1). Survey instruments show disturbed sleep and excessive sleepiness.2 PSG findings in humans with HD can be confounded by medications that change sleep architecture and may be difficult to perform because of chorea and dementia. However, the present case shares features of disordered sleep described in HD, including frequent awakenings/arousals, diminished REM, and lack of ultradian NREM‐REM cycles.1, 3, 4, 5 Other problems include delayed sleep‐onset latency, increased periodic limb movements, and REM behavior disorders.3, 4, 5 Although these reports emphasize associations with the primary degenerative process, the present case suggests that identification of common causes of arousals can lead to clinical benefits in HD.
The prevalence of nocturnal chorea in HD is unclear. Our findings agree with a previous study that found that chorea emerged during arousals.6 On the other hand, others found that, although motor activity was increased during sleep, chorea did not account for the preponderance of movements,5, 7 although diagnosing chorea by actigraphy7 may be difficult.
This is the first report of RBM in HD. RBM can fall into one of two nonexclusive categories. First, RBM may represent an element of hyperkinesia in HD, with chorea as the typical and RBM as the severe expression. However, in this patient, RBM was present before onset of chorea. On the other hand, RBM resembles rhythmic movement disorder, such as jactatio capitis nocturna (head banging).8 Though RBM is quasi‐rhythmic in morphology, we feel that the term “repetitive” is a more accurate descriptor of the movement than “rhythmic”. RBM shares with rhythmic movement disorder (1) a tendency for occurrence in those with cognitive impairment, (2) quasi‐rhythmic movements of 0.5 to 2.0 Hz, and (3) expression during both wakefulness and sleep‐wake transitions (although unlike head banging, RBM did not occur during sleep).
We speculate that because sleep is not well characterized in HD, nocturnal movement disorders may be more pervasive than recognized. What is clear, however, that for movements that are triggered by arousals, measures to diagnose reversible causes of sleep fragmentation can lead to effective treatment.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
S.R.: 1B, 1C, 2A
S.K.: 1C, 2A
M.B.H.: 1A, 1B, 2B
M.Q.: 1A, 2A, 2B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. Type A. Chorea after apnea‐induced arousal. Type B. Repetitive ballistic movement of the legs after awakening.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Morton AJ. Circadian and sleep disorder in Huntington's disease. Exp Neurol 2013;243:34–44. [DOI] [PubMed] [Google Scholar]
- 2. Videnovic A, Leurgans S, Fan W, Jaglin J, Shannon KM. Daytime somnolence and nocturnal sleep disturbances in Huntington disease. Parkinsonism Relat Disord 2009;15:471–474. [DOI] [PubMed] [Google Scholar]
- 3. Arnulf I, Nielsen J, Lohmann E, et al. Rapid eye movement sleep disturbances in Huntington disease. Arch Neurol 2008;65:482–488. [DOI] [PubMed] [Google Scholar]
- 4. Goodman AO, Rogers L, Pilsworth S, et al. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington's disease. Curr Neurol Neurosci Rep 2011;11:211–217. [DOI] [PubMed] [Google Scholar]
- 5. Silvestri R, Raffaele M, De Domenico P, et al. Sleep features in Tourette's syndrome, neuroacanthocytosis and Huntington's chorea. Neurophysiol Clin 1995;25:66–77. [DOI] [PubMed] [Google Scholar]
- 6. Fish DR, Sawyers D, Allen PJ, Blackie JD, Lees AJ, Marsden CD. The effect of sleep on the dyskinetic movements of Parkinson's disease, Gilles de la Tourette syndrome, Huntington's disease, and torsion dystonia. Arch Neurol 1991;48:210–214. [DOI] [PubMed] [Google Scholar]
- 7. Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep‐wake cycle and circadian timing in Huntington's disease. J Neurosci 2005;25:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thorpy MJ. Rhythmic movement disorder In: Thorpy MJ, ed. Handbook of Sleep Disorders. New York: Marcel Dekker; 1990:609–629. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. Type A. Chorea after apnea‐induced arousal. Type B. Repetitive ballistic movement of the legs after awakening.


