1. Abstract
Cancer therapies are known to alter the reproductive potential in cancer patients. Due to improved survival rates in cancer patients of reproductive age, considerations of the long-term effects of cancer therapy have become more significant. Oncofertility is a new discipline in medicine that deals with maintaining the reproductive potential of cancer patients while they are receiving gonadotoxic cancer treatment. The purpose of this review is to explore how cancer treatment impairs reproductive functioning and present the current options for preservation of fertility in women. All patients with reproductive potential should be made aware of the possible treatment-related infertility and be offered appropriate fertility preservation options before cancer treatment is instituted. The hope is that, in the future, mechanism(s) can be developed to preserve immature germ cells in the ovary, so that they can be used for fertilization in vivo or in vitro.
Keywords: Oncofertility, Fertility Preservation, Chemotherapy, Radiotherapy, Gonadotoxic, Cryopreservation, Follicles, Review
2. INTRODUCTION
It is estimated that approximately 1.6 million individuals were diagnosed with cancer in the United States in 2012; among these, 790,740 were women (2). Approximately 45% (almost one in two) of men and 38% (just over one in three) of women will be diagnosed with some form of invasive cancer during their lifetime. One in 134 individuals will develop some form of cancer at the age of 30. For a 45-year-old individual, the risk of developing cancer during the subsequent 10 years is 1 in 24 (1 in 26 for men; 1 in 22 for women) (3). As a result of significant advancements in current cancer therapy, the survival rate of cancer patients is continuing to improve. The overall five- year survival for females, aged 0–44, with malignant cancer diagnosed from 1999–2006 in the United States was 83.7% (4).
The marked improvement in survival as a result of early diagnosis, and newer and more targeted therapeutic approaches to malignant cancers, have resulted in a shift away from sole consideration of survivorship to the discussion of quality-of-life issues. A major effort is invested in the preservation of fertility and in maintaining the reproductive potential. This paradigm shift was termed oncofertility by Dr. Teresa Woodruff (5). Oncofertility refers to a new interdisciplinary field that bridges oncology and reproductive health research for the purpose of exploring and expanding options for maintaining the reproductive potential of patients.
Cancers of reproductive organs and systemic cancer treatment often threaten fertility in both sexes. In males, cryopreservation of semen is a safe and effective way of preserving fertility should treatment result in permanent sterility. In adult men with azoospermia on presentation, testicular sperm extraction may be successful at retrieving enough sperm for in vitro fertilization with intracytoplasmic sperm injection (ICSI). Currently, the options for fertility preservation in the prepubertal male, as well as female, are limited, and patients and families must be counseled that any procedure should be considered highly investigational at this time. Experimental work on the in vitro generation of sperm from harvested spermatogonial stem cells appears promising (6).
Female cancer patients have similar challenges of sub-fertility or infertility, but a significantly different and more restricted reproductive biology than males owing to the non-replenishable and finite number of ovarian follicles present from birth. The female germ cells (oocytes or eggs) must also be retrieved surgically and, while in limited numbers, they will also be in diverse states of maturity owing to the influence of the menstrual cycle (7). This is compounded by the fact that female gametes are more difficult to preserve. Female cancer patients may have impaired fertility due to cancer directly affecting their reproductive organs, thus requiring surgical excision or fertility loss may be the result of chemotherapy- or radiation-induced ovarian or uterine dysfunction.
Surveys of women diagnosed with cancer indicate that preservation of reproductive potential is of great importance to female cancer patients. Partridge et al. determined that 57% of breast cancer patients had substantial concerns about future fertility, and 29% reported that this concern influenced their decisions regarding treatment (8). The implication of this study is that women afflicted with cancer have added misgivings beyond the cancer itself that are also psychosocial in nature. Infertility resulting from cancer and/or its treatment has far-reaching consequences besides denying progeny in infertile women with cancer. Women with malignant disease report greater negative feelings and emotional distress, especially from unmet informational needs about reproductive options. They also report poorer sexual functioning (9). Impaired fertility, resulting from cancer and/or its treatment, compromises self-esteem, personal identity, sexuality, and self-image. Thus, infertility may fuel sentiments of emptiness and defeat. This can then negatively impact families and marriages. Conversely, fertility, or even the prospect thereof, fosters a sense of newness, life, hope, joy, pride, strength, optimism and purpose (10).
The purpose of this paper is to review known etiologies of fertility threats in female cancer patients and to examine current strategies for fertility preservation. The focus is on interventions aimed at preserving fertility in women undergoing gonadotoxic chemotherapy, radiotherapy, or oncological surgical procedures resulting in fertility compromise.
3. RADIOTHERAPY-ASSOCIATED DAMAGE
While radiation therapy is used to treat a myriad of cancers, ionizing radiation is a well-recognized cause of ovarian damage and infertility. The use of ionizing radiation results in atrophy of the ovary and reduced primordial follicle reserve. The estimated dose at which half of the follicles are lost in humans (LD50) is 4 Gy (11). The degree of ovarian damage caused by ionizing radiation is related to the patient’s age, dose of radiation used and trajectory of radiation as well as use of concomitant chemotherapy (12). Radiation treatment is administered by external-beam radiation therapy; it may also come from brachytherapy. Systemic radiation therapy uses radioactive substances, such as radioactive iodine, that travels in the blood to kill cancer cells (13). Abdominal, pelvic and spinal irradiation is associated with increased risk of developing acute ovarian failure, especially if both ovaries are within the treatment field. Direct actions on DNA are the predominant mechanism of damage for x-rays, gamma rays, photon or charged particle radiation such as neutrons. Indirect actions come from the interaction of radiation with other substances in the cell such as water leading to the formation of free radicals and DNA damage (14). Ovary damage can occur not only by direct exposure, but also by scatter radiation, even if the gonads are outside the radiation field. The ovaries of younger women are more resistant to permanent damage from irradiation due to the higher number of primordial follicles (15). In addition to causing follicular damage, radiotherapy also adversely impacts the uterus, rendering impaired uterine growth, uterine blood flow and decreased uterine volume. These uterine changes not only impact fertility, but also increase the risk for complications during pregnancy, such as early fetal loss, premature labor, and low birth weight (16).
4. CHEMOTHERAPY-ASSOCIATED DAMAGE
Chemotherapy is employed to treat many malignancies. Chemotherapies have different mechanisms of action and different gonadotoxic potentials. In general, chemotherapeutic drugs affect dividing cells. The conundrum, however, is that most human follicles, though primordial, do not undergo mitotic division. Nevertheless, they can still be damaged by chemotherapy.[0] The human ovary has a fixed, irreplaceable number of follicles that continue to undergo atresia during a woman’s reproductive life cycle until she reaches menopause (17). Compounding this loss is the fact that follicles are sensitive to the cytotoxic effects of chemotherapy, which leads to further follicle depletion. The exact mechanism by which chemotherapy damages follicles is unknown. Meirow et al. posit possible mechanisms to include follicular apoptosis, or follicular “burn out,” as a hypothesis. It is suggested that there is an increased activation of follicles from the resting pool, resulting in accelerated atresia, and premature burn- out of the primordial follicle reserve. In addition, cortical fibrosis may occur where anticancer therapy leads to hyalinization of cortical blood vessels, neovascularization, and cortical fibrosis, which results in local ischemia impairing growth and survival of primordial follicles (18). Evidence reveals that anticancer drugs reduce the primordial follicle pool, promote ovarian atrophy, and limit ovarian blood vasculature (19). Histological ovarian specimens reveal ovarian atrophy with diminished primordial follicle reserve, decreased ovarian weight, and stromal fibrosis (20).
A direct measure of ovarian reserve is not possible, but a crude estimate may be performed via ultrasound-guided estimation of ovarian volume, antral follicle count, and determining levels of anti-Mullerian hormone (AMH) and inhibin B (21). The degree of ovariotoxicity depends on the drug, dosage of drug, use of concomitant chemotherapies or radiation, method of administration, and the disease process being treated as well as the age, and pretreatment fertility of the patient (22). There is recent evidence that advanced maternal age is associated with deterioration of chromosome cohesion due to increased inter-kinetochore distances between sister chromatids and chromosome segregation errors in meiosis I, an explanation for age-related poor egg quality due to aneuploidy (23).
The greatest risk of ovarian damage signified by amenorrhea is associated with treatment involving alkylating agents (notably cyclophosphamide, ifosfamide, nitrosoureas, chlorambucil, melphalan, busulfan, and procarbazine), one of the six main classes of chemotherapeutic drugs (24). Cyclophosphamide is a drug used to treat many solid tumors as well as leukemias and lymphomas. It is a member of the nitrogen mustard family and works by attaching an alkyl group to the guanine base of DNA. While cyclophosphamide is the chemotherapy most often noted for damaging oocytes and granulosa cells in a dose-dependent manner, it is not cell cycle specific and, therefore, able to damage cells at different stages of the cell cycle, including resting primordial follicles (25, 26).
Other classes of chemotherapeutic drug groups include platinum derivatives, antibiotics, antimetabolites, plant alkaloids, and the taxanes. Platinum derivatives, such as cisplatin, have also been shown to be ovariotoxic with an estimated odds ratio (OR) of 1.77 for ovarian failure (19). The effects of newer targeted therapies, such as monoclonal antibody therapy with bevacizumab, or epothilone agents, such as ixabepilone, are not known. As these targeted therapies become more widely used, it will be easier to assess their effects on oncofertility.
There are few studies performed on human ovaries detailing the impact of chemotherapeutic drugs (Table 1) (27–32). More studies will need to be undertaken to determine how cancer itself and chemotherapies affect chromosome dynamics during meiosis as these eggs may be fertilized or cryopreserved for fertility preservation.
Table 1.
Effects of Chemotherapy on human folliculogenesis and ovary
| Chemotherapeutic agent |
Dosage | Effects of chemotherapeutic agent | References |
|---|---|---|---|
| Doxorubicin | 100 µg/ml for 24 and 72h (in vitro) |
Apoptosis and decline in ovarian density, affected primordial, pre-antral follicles, oocytes, granulosa cells, stroma, blood vessels |
27 |
| Cyclophosphamide | A single dose of 200 mg/kg for 48 h |
Apoptosis in primordial follicles, oocytes, granulosa cells | 28 |
| Cyclophosphamide | (0.5 mg/mL) for 2–48 h | Damaged granulosa cell nuclei in human ovarian cortical slices | 29 |
| Cyclophosphamide, carmustine |
(see reference for details) | Deterioration in pre-antral follicles quality with abnormal granulosa cell nuclei | 30 |
| Non-sterilizing doses of alkylating and non- alkylating chemotherapy |
(see reference for details) | Damage to cortical blood vessel and proliferation of small vessels. The cortex showed focal areas of fibrosis with disappearance of follicles |
31 |
| Alkylating regimens and non-alkylating regimens |
(see reference for details) | Regimens containing alkylating agents resulted in significant loss of ovarian reserve affecting primordial follicles; however, non-alkylating chemotherapy regimens also appeared to alter ovarian stromal function evidenced by decreased estradiol production |
32 |
5. CURRENT METHODS FOR FERTILITY PRESERVATION
While chemotherapy or radiation may lead to reduced ovarian reserve or ovarian failure, there are a limited number of established and reliable approaches to preserving fertility encompassing pharmacological, surgical, and investigational treatments with promising results (Figure 1).
Figure 1.
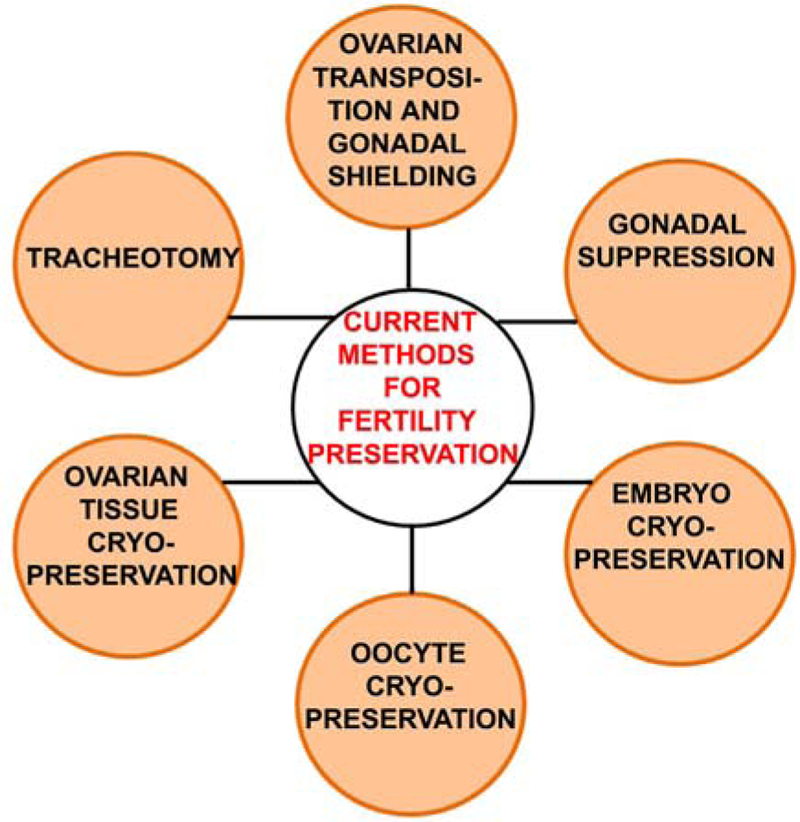
A schematic representation of current methods of oncofertility preservation (see details in text).
5.1. Ovarian transposition and gonadal shielding
Pelvic irradiation is a common treatment for cancer of the lower genital and intestinal tract, urinary tract, and some Hodgkin’s lymphomas. Mitigating the effects of pelvic irradiation, which can cause ovarian follicle depletion and uterine damage resulting in pregnancy loss, premature labor, or intrauterine growth restriction, is of paramount importance in preserving fertility. Two well- established techniques in use include ovarian transposition and gonadal shielding.
Ovarian transposition, also known as oophoropexy or ovarian suspension, is an outpatient surgical procedure, which is performed before radiation therapy and whereby the ovaries are moved out of the radiation field at least to the level above the pelvic brim (33). The ovaries may be repositioned in a subsequent surgery or in vitro fertilization may be performed to conceive. Spontaneous pregnancies have occurred without repositioning the ovaries back to their original location. The ovaries do not have to be repositioned unless the patient is unable to conceive (34). The ovarian suspension may be done laparoscopically or by laparotomy and rates of ovarian preservation in women less than 40 years of age have been as high as 90% (35). This technique reduces radiation exposure to the ovaries to only 5 to 10% of what has been reported in ovaries that were not suspended above the pelvic brim (36). The reported success rates are discordant, ranging between 16 and 90% (37). Failures are due to such factors as vascular compromise, scatter radiation, radiation dose, patient age, and whether the ovaries are shielded during the radiation procedure (38). Complications of ovarian transposition have included chronic ovarian pain, and reports of metastasis to the transposed ovaries and to port sites.
Gonadal shielding is a technique whereby bismuth or lead shields are applied externally to the gonads, whenever possible, to minimize their exposure to radiation (39). Gonadal shielding may be used in conjunction with ovarian transposition during radiation therapy to reduce the dose of radiation delivered to the reproductive organs. Expertise is required to ensure that the shielding does not increase the dose delivered to reproductive organs.
5.2. Gonadal suppression
The use of hormone suppression for protecting gonadal function after cytotoxic exposure is based on the observation that non-cycling cells are generally more resistant to killing by certain toxicants, particularly antineoplastic agents, than are rapidly proliferating cells. The greater sensitivity of cycling as compared to non- cycling cells is the basis for the antitumor action of many of these antineoplastic agents (40). Combined hormone contraceptives were the first hormonal method to be tried. Despite some encouraging results, follicular growth was not completely inhibited (41–44). Ovarian suppression with gonadotropin-releasing hormone analogs (GnRHa) is the mainstay of hormonal therapies used to protect ovarian tissue during chemotherapy (45).
The exact mechanism by which GnRHa protects the ovary during chemotherapy remains inconclusive. It has been postulated that GnRHa protects the ovary by inhibiting the hypothalamic-pituitary- ovarian (HPO) axis and inducing a prepubertal state. Normally, human GnRH is released in a pulsatile fashion, stimulating gonadotropin release that actuates the ovulatory cycle and subsequent ovarian steroidogenesis. With the administration of synthetic GnRH agonists, the surge in GnRH floods the pituitary GnRH receptors, ultimately causing the pituitary GnRH receptors to be downregulated, and gonadotropin release to be prevented. This inhibits the pituitary-ovarian cycle, resulting in pre-pubertal levels of estrogen (46).
Another route by which GnRHa may provide ovarian protection is via a reduction in ovarian blood flow. This causes a decrease in the amount of chemotherapy reaching the ovary, which has been inferred from the fact that uterine blood flow has been shown to be reduced after administration of GnRHa (47). Other reports did not detect a difference in ovarian stromal blood flow before and after GnRHa pituitary downregulation for in vitro fertilization (48, 49).
A direct effect of GnRHa on the ovary has also been proposed. While GnRH receptors have been identified in ovarian cancer cell lines, ovarian surface epithelium, preovulatory follicles, and the corpus luteum, they have not been identified in primordial or early antral follicles (50). This precludes a mechanism by which GnRHa would protect the primordial and preantral follicles that make up the majority of the follicle pool and lack GnRH receptors.
The mechanisms underlying the protective nature of GnRHa need further clarification. Blumenfeld et al. determined that co-administration of GnRHa with chemotherapy in 65 women for six months versus 46 others with chemotherapy alone led to the return of regular menses and ovulation in 96.9% of women in the GnRHa- treated group and 63% in the control group (51). Megan et al. conducted a meta-analysis that showed that GnRHa therapy increases the chances of maintaining ovarian function and childbearing by 65%–68% over chemotherapy alone (52). Yet, Waxman et al. conducted a randomized study of 30 men and 18 women with Hodgkin’s disease, who received GnRHa prior to and for the duration of chemotherapy and found it to be ineffective in preserving fertility (53).
The use of GnRHa in patients undergoing chemotherapy is controversial. Randomized controlled trials have demonstrated no benefit in clinical pregnancy rates, but the return of ovulation and menses is higher in female cancer patients who received GnRHa (54). The American Society of Clinical Oncology (ASCO) states that there is insufficient evidence that GnRHa protects gonadal function from gonadotoxic agents and that the safety and effectiveness, especially in women with hormone sensitive tumors, warrants further investigation and urges women interested in ovarian suppression to participate in clinical trials (55). Gonadal suppression with GnRHa is an option that may be discussed with patients, but its success is disputable and the side effects related to hypoestrogenism are considerable.
5.3. Embryo cryopreservation
Embryo cryopreservation has been available to patients since 1983 (56). It is the most established technique for fertility preservation in women. The first child to be born as a result of embryo freezing was in 1984 (57). It entails a woman undergoing a superovulation regimen with FSH and hCG injections, serial ultrasounds and blood tests to monitor follicle development, followed by abstraction of oocytes, in vitro fertilization, and freezing of the embryos for later implantation once cancer therapy has been completed. This technique requires 10–14 days of ovarian stimulation from the beginning of the menstrual cycle, followed by an outpatient surgical procedure for oocyte retrieval, and requires a sexually mature woman with a partner or donor sperm (55).Embryo cryopreservation is not suitable for children or young adults without a partner and those who do not desire to use donor sperm. In addition, this technique requires that cancer therapy be suspended for at least two weeks to allow for hormonal stimulation and oocyte retrieval, with the ensuing delay in initiation of therapy (58). In women with hormone-sensitive malignancies, such as breast, endometrial or ovarian cancers, there is the additional concern regarding the safety of ovarian stimulation with respect to the high estradiol levels attained (59).
5.4. Oocyte vitrification / cryopreservation
Oocyte cryopreservation is the harvesting and freezing of unfertilized mature eggs from reproductive aged women. Oocyte cryopreservation comprises both freezing and vitrification. Oocyte freezing is a technique that arose in tandem with embryo cryopreservation. The first live birth as a result of oocyte freezing was in 1986 (60). The technique of oocyte cryopreservation, however, remained clinically-dormant with fewer than 150 pregnancies prior to 2004 due to technical challenges (61).
Oocyte freezing involves gradually exposing oocytes to relatively low concentrations of permeating cryoprotectants (CPs), such as glycerol, DMSO, ethylene glycol or propylene glycol and non-permeating factors such as sucrose, glucose, or fructose, which are added to the culture medium. The oocytes are then loaded in small volumes into straws and cooled to about −5 to −7 °C where they are kept for several minutes to equilibrate. After equilibration, the solution is cooled slowly and progressively, at about 0.3–0.5 °C/min, to anywhere between −30 and −65 °C. Once the desired temperature is attained, the straws are plunged into liquid nitrogen for storage (62).
Vitrification is another type of cryopreservation technique and which consists of the ultra-rapid freezing of cells, turning their intra- and extracellular aqueous environment into a glassy-like state. Vitrification combines two different biophysical processes: a preliminary equilibration step in which oocytes are exposed to low concentrations of cryoprotectants to allow water outflow, and a subsequent vitrification phase in which cells undergo a high osmotic gradient that completes cells’ dehydration. Under these conditions, oocytes can be directly merged into liquid nitrogen and then stored. Similarly, warming of oocytes must be rapid in order to avoid recrystallization of water. The CPs used during vitrification are the same employed for slow freezing, but they are three-to-four-fold more concentrated in vitrification than in slow freezing (63).
Noyes et al. determined in 2008 that with significant improvements made in techniques of oocyte preservation, there have been more than 900 live births worldwide, of which an estimated 500 live births from oocyte preservation occurred in the United States alone (64, 65).
Like embryo cryopreservation, oocyte cryopreservation requires 10–14 days of ovarian stimulation from the beginning of the menstrual cycle and outpatient surgery to retrieve oocytes. This method of fertility preservation is suitable for reproductive- aged women without a partner, but pregnancy rates are diminished because oocytes are more susceptible to damage during the freeze-thawing process than the resilient cryopreserved embryos. Oocytes have high intracellular water content that leads to ice crystal formation that can lead to cellular damage.
5.5. Ovarian tissue cryopreservation
Ovarian cryopreservation and transplantation is the freezing of ovarian tissue after surgically removing it. It is later reimplanted, either orthotopically (pelvis) or heterotopically (forearm, abdomen), after cancer treatment to protect and restore reproductive function in female cancer patients receiving cytotoxic chemotherapy and/or radiotherapy (66). This is not a suitable preservation option when the risk of ovarian cancer involvement is high, such as with systemic hematologic malignancies. It requires a surgical procedure to obtain ovarian tissue and a subsequent procedure to reimplant it.
The advantage of ovarian tissue cryopreservation is that there is no delay, as the tissue can be obtained without ovarian stimulation and no partner is required at the time of tissue removal. Once the tissue is transplanted, it will resume production of endogenous hormones. Returning the ovarian tissue back to the original ovarian site is known as orthotopic transplantation. The graft risks ischemic compromise because it takes 48–72 hours to grow a new microscopic blood supply. During this ischemic time, the oocytes may be destroyed. The functional life span of transplanted tissue is reported to be three years due to follicle attrition attributed to ischemic injury (67). Alternatively, heterotopic transplantation entails the ovarian tissue being grafted subcutaneously at various locations, including the forearm or abdominal wall. Heterotopic transplantation is the preferred method of monitoring the ovaries when there is concern for malignant transformation. There is currently research being conducted in the arena of cryopreservation of whole ovaries with vascular anastomosis (68). With this method of fertility preservation, there exists a theoretical risk of reimplanting metastatic cells originating from the primary tumor. While considered experimental, fresh and frozen cortical ovarian tissue transplantations have been successfully reported worldwide resulting in approximately 28 healthy babies (69).
5.6. Trachelectomy
Resection of the cervix while preserving the uterine fundus was first performed by Aburel in the late 1930s (70). In the United States, the median age at diagnosis of cervical cancer is 48 years (71). It is estimated that 43% of all cases of cervical cancer in the United States are diagnosed in women younger than 45 years of age (72). Sonoda et al. determined that approximately 48% of women with operable early-stage cervical cancer may be eligible for fertility-sparing treatment (73). Approximately 3000 cases of cervical cancer will occur in premenopausal women per annum (74). Traditionally, cervical cancer is treated either surgically with radical hysterectomy or with radiation therapy. Yet, neither option allows continued function of the utero-ovarian system necessary for reproduction. Trachelectomy may be performed vaginally or abdominally; its use is limited to female patients with early stage cervical cancer who desire future fertility. Kim et al. found a 66% success rate in conceiving with 35% delivering between 32 and 36 weeks and 65% delivering at ≥ 37 weeks of gestation (75). The pregnancy complications faced by women who have undergone a trachelectomy include increased risk of cervical incompetence, preterm premature rupture of membranes and preterm delivery. Recent reviews of the published literature totaling more than 600 trachelectomy cases confirm an overall cancer recurrence rate of < 5% for radical trachelectomy and a death rate of < 3%, which is consistent with results of radical hysterectomies for similar-sized lesions (76).
6. DEVELOPMENT OF THE OVARIAN FOLLICLE
Folliculogenesis, or the development of the ovarian follicle, is a complex process that involves autocrine and paracrine mediators, internal and extraovarian hormonal mediators, and interaction of somatic cell components and the female gamete, the oocyte (77). At birth, the human ovary contains approximately one million primordial follicles, each containing an oocyte in meiotic arrest at the prophase stage (78).The oocyte is surrounded by a layer of somatic granulosa cells. Follicular growth from the primordial to the pre-ovulatory stage occurs in two distinct stages. The first growth phase is gonadotropin-independent and the second stage is gonadotropin-dependent (79).
The primordial follicle pool represents the largest pool of follicles in the ovary, making them prime targets for use in association for fertility preservation. While it makes logical sense to consider the primordial follicles as primary candidates for preservation of fertility, the emerging technology is challenged by a number of factors. Normal follicular growth spanning from primordial to ovulatory follicle stages is a protracted process. Gougeon estimated that the growth phase from primordial to primary follicle in humans takes > 120 days. Once in the growing pool, the follicle requires 65 days to reach the early antral phase (follicle of 2–5 mm diameter). At this point, it becomes dependent on gonadotropins for further growth (80). In addition, given that the follicles mature through several developmental stages, the trophic requirements also change evidenced by the fact that in vivo folliculogenesis is mediated by a complex interplay of gonadotropic hormones as well as peptide growth factors (81–83). Encouragingly, in vitro rodent follicle culture has proved successful (84). The challenge with humans is that it is technically more difficult to recover the follicles since their ovarian cortex is dense and fibrous and the follicles grow to a considerable size (85).
6.1. In vitro ovarian follicle culture
In vitro follicle culture is the newest horizon in assisted reproductive technology. The ovarian cortex contains numerous primordial follicles, which envelop immature oocytes in meiotic arrest. However, nature, via hormonal influences, directs a series of developmental stages, triggers the maturation of a single primordial follicle to the Graafian stage and results in the ovulation of a single mature oocyte. Currently, in vitro fertilization treatment involves the administration of hormone injections to stimulate the maturation of multiple follicles within the ovary. These mature oocytes can then be extracted and fertilized or frozen. Yet, this maturation process is limiting because it can only be applied to reproductively-mature women. An alternative approach is to extract ovarian tissue containing immature follicles and induce growth and maturation of oocytes in vitro. The limit to ovarian and follicle cryopreservation has been the ability to induce in vitro maturation of the follicle-oocyte complex in the laboratory (86).
After overcoming the mechanical isolation challenge from a fibrous cortex with enzymatic digestion, it is important to maintain the follicle unit’s integrity and ultrastructure. At each stage, the maturing oocyte is dependent on the follicular granulosa cell layers. Effective communication between the oocyte and the surrounding somatic supportive cellular network is paramount for oocyte survival and maturation and is achieved via gap junctions, which allow cross-talk between the somatic cells of the follicle and the oocyte.
In addition to the cell-cell communication, the spatial arrangement of follicles is impacted by the type of culture system, whether it is 2-D (two-dimensional) or 3-D (three-dimensional). Two-dimensional systems are less optimal for culture because of their inability to maintain spatial arrangements of cells seen in vivo. In the traditional 2-D culture systems, the follicles flatten and lose their 3-D spatial arrangement as granulosa cells attach to the culture plates (87). The in vivo spherical structure is lost and pertinent communication links between the oocyte and the surrounding somatic cells become disrupted, thereby impeding oocyte maturation. A 3-D culture more closely resembles follicles in vivo by preserving cellular spatial arrangements, behavior, growth, secretions, response to stimuli and communication with surrounding cells. Not only is conservation of spatial arrangement important, but evidence now points to the extracellular matrix (ECM) exerting a role in organizing communication between cells, controlling cell differentiation and modifying responses to autocrine and paracrine mediators from the cellular environment. Three-dimensional culture maintains the integrity of cellular signaling seen in vivo (88).
Many biologic and synthetic matrices have been evaluated to determine whether they support follicle growth and oocyte maturation. The predominant candidate is calcium alginate, which is produced by brown algae. Alginate forms crosslinks in the presence of calcium to form a hydrogel. It is thought to mimic the stromal microenvironment of the ovary. Reports have demonstrated that murine follicles grown in alginate capsules reach sizes typically observed in vivo (89).
While the technology of in vitro growth and maturation of follicles and oocyte, respectively, is promising, numerous questions and technical difficulties still remain. This includes optimization of the pH, temperature and oxygen tension of the culture environment to maximally promote follicle and oocyte development and minimize apoptosis. There is also a need to evaluate the effect of long-term culture on the epigenetic information and methylation status of imprinted genes in oocytes (90). In humans, in vitro culture and .
Successful culture of primordial or early stage human follicles will likely require development of a multipronged approach. Picton et al. devised four steps to optimize in vitro maturation of human follicles: phase 1 with in situ culture of primordial and primary follicles, followed by phase 2 after reaching the secondary stage of development, whereby preantral and antral follicles are isolated and encapsulated. Phase 2 aims to support antral cavity formation as well as oocyte growth to full size. Phase 3 will require induction of differentiated function in follicular somatic cells mediated by steroid hormones and gonadotropins so that the oocyte obtains oocyte cytoplasmic competence and phase 4 will culminate with the in vitro maturation of oocytes. This allows for the maturation of a fully-grown, cumulus-enclosed, germinal vesicle stage oocyte in meiotic progression to metaphase II (91).
The scientific obstacle this technology hopes to eliminate is the current inability to grow immature human follicles and support the entire development of the egg through terminal meiotic maturation of the oocyte in vitro, so it may be fertilized and transferred to the female recipient (92). Viable, healthy human follicles have been grown in vitro for 30 days (93). This holds tremendous promise in in vitro follicle maturation of human follicles once the technique is refined. Fertility preservation will have far-reaching potential irrespective of the reproductive stage of a female, offering expanded fertility preservation options even to pre-pubertal females.
7. CONCLUSIONS
Oncofertility constitutes an emerging and rapidly expanding branch of health research. Heretofore embryo and oocyte cryopreservation were the only successful methods for women with impaired fertility resulting from premature ovarian failure and an inability to achieve pregnancy. Currently, there are viable options, albeit experimental, which are widening the arsenal. These include cryopreservation of mature and in vitro-matured oocytes, embryos, and ovarian tissue. Furthermore, there are advances in technology leading to the promise of culturing primordial follicles obtained from ovarian tissue and maturing them in vitro. This will provide an abundant supply of oocytes. In the interim, as this technology is perfected, a female patient will require transplantation of tissue or relocalization of ovaries if undergoing radiotherapy. As the field of oncofertility advances, increased knowledge of drugs and attempts to mitigate chemotoxicity, as well as information-sharing between practitioners and patients, will be required to promote a better understanding of fertility preservation options.
8. ACKNOWLEDGEMENTS
The authors thank Ernest A. Alema-Mensah, Ph.D. for statistical assistance in the preparation of this manuscript. The authors also thank Angela D. Wimes, M.A. for her editorial assistance in the preparation of this manuscript. This work was supported by the National Institutes of Health, CRECD Grant #2R25RR017694–06A, SC11SC1HL095099 and Grant #8G12MD007602 from the National Institute of Minority Health and Health Disparities (NIMHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities (NIMHD) or the National Institutes of Health (NIH). The authors have no conflicts of interest to disclose.
9. REFERENCES
- 1.Trost LW and Brannigan RE: Oncofertility and the male cancer patient. Curr Treat Options Oncol, 13(2), 146– 60 (2012) [DOI] [PubMed] [Google Scholar]
- 2.A. C. Society.: Cancer Facts & Figures 2012 (2012)
- 3.Hayat MJ, Howlader N, Reichman ME and Edwards BK: Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist, 12(1), 20–37 (2007) [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF KC, Krapcho M, et al. ,: SEER cancer statistics review, 1975–2007 (2009. )
- 5.Woodruff TKKAS: Oncofertility: Fertility Preservation for Cancer Survivors (Chicago: Springer; ) (2007) [Google Scholar]
- 6.Holoch P and Wald M: Current options for preservation of fertility in the male. Fertil Steril, 96(2), 286–90 (2011) [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SK and Chang RJ: Fertility management for women with cancer. Cancer Treat Res, 138, 15–27 (2007) [DOI] [PubMed] [Google Scholar]
- 8.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A and Winer EP: Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol, 22(20), 4174–83 (2004) [DOI] [PubMed] [Google Scholar]
- 9.Carter J, Raviv L, Applegarth L, Ford JS, Josephs L, Grill E, Sklar C, Sonoda Y, Baser RE and Barakat RR: A cross-sectional study of the psychosexual impact of cancer-related infertility in women: third-party reproductive assistance. J Cancer Surviv, 4(3), 236–46 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tschudin S and Bitzer J: Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update, 15(5), 587–97 (2009) [DOI] [PubMed] [Google Scholar]
- 11.Wallace WH, Shalet SM, Hendry JH, Morris-Jones PH and Gattamaneni HR: Ovarian failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol, 62(743), 995–8 (1989) [DOI] [PubMed] [Google Scholar]
- 12.Meirow D and Nugent D: The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update, 7(6), 535–43 (2001) [DOI] [PubMed] [Google Scholar]
- 13.Lawrence TS THR, Giaccia A : Cancer: Principles and Practice of Oncology, 8th ed. (Lippincott Williams and Wilkins; ) (2008) [Google Scholar]
- 14.The Final Report of the Advisory Committee on Human Radiation Experiments(http://hss.energy.gov/healthsafety/ohre/roadmap/achre/intro_9_5.html) [DOI] [PubMed]
- 15.Wallace WH, Thomson AB, Saran F and Kelsey TW: Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys, 62(3), 738–44 (2005) [DOI] [PubMed] [Google Scholar]
- 16.Critchley HO, Bath LE and Wallace WH: Radiation damage to the uterus – review of the effects of treatment of childhood cancer. Hum Fertil (Camb), 5(2), 61–6 (2002) [DOI] [PubMed] [Google Scholar]
- 17.Reynaud K and Driancourt MA: Oocyte attrition. Mol Cell Endocrinol, 163(1–2), 101–8 (2000) [DOI] [PubMed] [Google Scholar]
- 18.Meirow D, Biederman H, Anderson RA and Wallace WH: Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol, 53(4), 727–39 (2010) [DOI] [PubMed] [Google Scholar]
- 19.Meirow D: Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol, 169(1–2), 123–31 (2000) [DOI] [PubMed] [Google Scholar]
- 20.Nicosia SV, Matus-Ridley M and Meadows AT: Gonadal effects of cancer therapy in girls. Cancer, 55(10), 2364–72 (1985) [DOI] [PubMed] [Google Scholar]
- 21.Anderson RA and Cameron DA: Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab, 96(5), 1336–43 (2011) [DOI] [PubMed] [Google Scholar]
- 22.Committee IP, Kim SS, Donnez J, Barri P, Pellicer A, Patrizio P, Rosenwaks Z, Nagy P, Falcone T, Andersen C, Hovatta O, Wallace H, Meirow D, Gook D, Kim SH, Tzeng CR, Suzuki S, Ishizuka B and Dolmans MM: Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet, 29(6), 465–8 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD and Woodruff TK: Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell, 11(6), 1121–4 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waxman J: Chemotherapy and the adult gonad: a review. J R Soc Med, 76(2), 144–8 (1983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenney LB, Laufer MR, Grant FD, Grier H and Diller: High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer, 91(3), 613–21 (2001) [DOI] [PubMed] [Google Scholar]
- 26.Koyama H, Wada T, Nishizawa Y, Iwanaga T and Aoki Y: Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer, 39(4), 1403–9 (1977) [DOI] [PubMed] [Google Scholar]
- 27.Soleimani R and De Sutter P: In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod, 26(4), 955–6; author reply 956–9 (2011) [DOI] [PubMed] [Google Scholar]
- 28.Oktem O and Oktay K: A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res, 67(21), 10159–62 (2007) [DOI] [PubMed] [Google Scholar]
- 29.Raz A, Fisch B, Okon E, Feldberg D, Nitke S, Raanani H and Abir R: Possible direct cytoxicity effects of cyclophosphamide on cultured human follicles: an electron microscopy study. J Assist Reprod Genet, 19(10), 500–6 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abir R B-HA, Felz C, Okon E, Raanani H, Orvieto R, Nitke S, Fisch B: Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum Reprod 23(4), 869–77 (2008. ) [DOI] [PubMed] [Google Scholar]
- 31.Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, Raanani H, Levron J and Fridman E: Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod, 22(6), 1626– 33 (2007) [DOI] [PubMed] [Google Scholar]
- 32.Oktem O and Oktay K: Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer, 110(10), 2222–9 (2007) [DOI] [PubMed] [Google Scholar]
- 33.Kurt M, Uncu G, Cetintas SK, Kucuk N, Guler S and Ozkan L: Successful spontaneous pregnancy in a patient with rectal carcinoma treated with pelvic radiotherapy and concurrent chemotherapy: the unique role of laparoscopic lateral ovary transposition. Eur J Gynaecol Oncol, 28(5), 408–10 (2007) [PubMed] [Google Scholar]
- 34.Thomas PR, Winstanly D, Peckham MJ, Austin DE, Murray MA and Jacobs HS: Reproductive and endocrine function in patients with Hodgkin’s disease: effects of oophoropexy and irradiation. Br J Cancer, 33(2), 226–31 (1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisharah M and Tulandi T: Laparoscopic preservation of ovarian function: an underused procedure. Am J Obstet Gynecol, 188(2), 367–70 (2003) [DOI] [PubMed] [Google Scholar]
- 36.Sonmezer M and Oktay K: Fertility preservation in female patients. Hum Reprod Update, 10(3), 251–66 (2004) [DOI] [PubMed] [Google Scholar]
- 37.Sonmezer M, Oktay K: Fertility preservation in patients undergoing gonadotoxic treatment or gonadal resection UpToDate (2013)
- 38.Nakagawa K, Kanda Y, Yamashita H, Hosoi Y, Oshima K, Ohtomo K, Ban N, Yamakawa S, Nakagawa S and Chiba S: Preservation of ovarian function by ovarian shielding when undergoing total body irradiation for hematopoietic stem cell transplantation: a report of two successful cases. Bone Marrow Transplant, 37(6), 583–7 (2006) [DOI] [PubMed] [Google Scholar]
- 39.Falcone T, Attaran M, Bedaiwy MA and Goldberg JM: Ovarian function preservation in the cancer patient. Fertil Steril, 81(2), 243–57 (2004) [DOI] [PubMed] [Google Scholar]
- 40.Meistrich ML and Shetty G: Hormonal suppression for fertility preservation in males and females. Reproduction, 136(6), 691–701 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai A and Furui T: Chemotherapy-induced female infertility and protective action of gonadotropin-releasing hormone analogues. J Obstet Gynaecol, 27(1), 20–4 (2007) [DOI] [PubMed] [Google Scholar]
- 42.Chapman RM and Sutcliffe SB: Protection of ovarian function by oral contraceptives in women receiving chemotherapy for Hodgkin’s disease. Blood, 58(4), 849–51 (1981) [PubMed] [Google Scholar]
- 43.Longhi A, Pignotti E, Versari M, Asta S and Bacci G: Effect of oral contraceptive on ovarian function in young females undergoing neoadjuvant chemotherapy treatment for osteosarcoma. Oncol Rep, 10(1), 151–5 (2003) [PubMed] [Google Scholar]
- 44.Whitehead E, Shalet SM, Blackledge G, Todd I, Crowther D and Beardwell CG: The effect of combination chemotherapy on ovarian function in women treated for Hodgkin’s disease. Cancer, 52(6), 988–93 (1983) [DOI] [PubMed] [Google Scholar]
- 45.Blumenfeld Z and von Wolff M: GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update, 14(6), 543–52 (2008) [DOI] [PubMed] [Google Scholar]
- 46.Periti P, Mazzei T and Mini E: Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet, 41(7), 485–504 (2002) [DOI] [PubMed] [Google Scholar]
- 47.Reinsch RC, Murphy AA, Morales AJ and Yen SS: The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: a prospective, randomized study. Am J Obstet Gynecol, 170(6), 1623–7; discussion 1627–8 (1994) [PubMed] [Google Scholar]
- 48.Ng EH, Tang OS, Chan CC and Ho PC: Ovarian stromal blood flow in the prediction of ovarian response during in vitro fertilization treatment. Hum Reprod, 20(11), 3147–51 (2005) [DOI] [PubMed] [Google Scholar]
- 49.Jarvela IY, Sladkevicius P, Kelly S, Ojha K, Campbell S and Nargund G: Effect of pituitary down- regulation on the ovary before in vitro fertilization as measured using three-dimensional power Doppler ultrasound. Fertil Steril, 79(5), 1129–35 (2003) [DOI] [PubMed] [Google Scholar]
- 50.Choi JH, Gilks CB, Auersperg N and Leung PC: Immunolocalization of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and type I GnRH receptor during follicular development in the human ovary. J Clin Endocrinol Metab, 91(11), 4562–70 (2006) [DOI] [PubMed] [Google Scholar]
- 51.Blumenfeld Z, Avivi I, Eckman A, Epelbaum R, Rowe J and Dann EJ: Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril, 89(1), 166–73 (2008) [DOI] [PubMed] [Google Scholar]
- 52.Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC and Bastian LA: Ovarian preservation by GnRH agonists during chemotherapy: a meta- analysis. J Womens Health (Larchmt), 18(3), 311–9 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waxman JH, Ahmed R, Smith D, Wrigley PF, Gregory W, Shalet S, Crowther D, Rees LH, Besser GM, Malpas JS and et al. : Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol, 19(2), 159–62 (1987) [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Li J, Cui T and Hu L: Adjuvant gonadotropin- releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev(11), CD008018 (2011) [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K and American O Society of Clinical: American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol, 24(18), 2917–31 (2006) [DOI] [PubMed] [Google Scholar]
- 56.Trounson A and Mohr L: Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature, 305(5936), 707–9 (1983) [DOI] [PubMed] [Google Scholar]
- 57.Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM and Drogendijk AC: Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril, 42(2), 293–6 (1984) [DOI] [PubMed] [Google Scholar]
- 58.Baynosa J, Westphal LM, Madrigrano A and Wapnir I: Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg, 209(5), 603–7 (2009) [DOI] [PubMed] [Google Scholar]
- 59.Stern CJ, Toledo MG, Gook DA and Seymour JF: Fertility preservation in female oncology patients. Aust N Z J Obstet Gynaecol, 46(1), 15–23 (2006) [DOI] [PubMed] [Google Scholar]
- 60.Chen C: Pregnancy after human oocyte cryopreservation. Lancet, 1(8486), 884–6 (1986) [DOI] [PubMed] [Google Scholar]
- 61.Stachecki JJ and Cohen J: An overview of oocyte cryopreservation. Reprod Biomed Online, 9(2), 152–63 (2004) [DOI] [PubMed] [Google Scholar]
- 62.Saragusty J and Arav A: Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction, 141(1), 1–19 (2011) [DOI] [PubMed] [Google Scholar]
- 63.Revelli A, Molinari E, Salvagno F, Delle Piane L, Dolfin E and Ochetti S: Oocyte cryostorage to preserve fertility in oncological patients. Obstet Gynecol Int, 2012, 525896 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noyes N, Porcu E and Borini A: Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online, 18(6), 769– 76 (2009) [DOI] [PubMed] [Google Scholar]
- 65.Rudick B, Opper N, Paulson R, Bendikson K and Chung K: The status of oocyte cryopreservation in the United States. Fertil Steril, 94(7), 2642–6 (2010) [DOI] [PubMed] [Google Scholar]
- 66.Practice M Committee of the American Society for Reproductive: Ovarian tissue and oocyte cryopreservation. Fertil Steril, 82(4), 993–8 (2004) [DOI] [PubMed] [Google Scholar]
- 67.Newton H, Aubard Y, Rutherford A, Sharma V and Gosden R: Low temperature storage and grafting of human ovarian tissue. Hum Reprod, 11(7), 1487–91 (1996) [DOI] [PubMed] [Google Scholar]
- 68.Zhang JM, Sheng Y, Cao YZ, Wang HY and Chen ZJ: Effects of cooling rates and ice-seeding temperatures on the cryopreservation of whole ovaries. J Assist Reprod Genet, 28(7), 627–33 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R and Frydman N: Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril, 97(6), 1260–8 (2012) doi: 10.1016/j.fertnstert.2012.04.042 [DOI] [PubMed] [Google Scholar]
- 70.Rasool N and Rose PG: Fertility-preserving surgical procedures for patients with gynecologic malignancies. Clin Obstet Gynecol, 53(4), 804–14 (2010) [DOI] [PubMed] [Google Scholar]
- 71.N. A.Howlader N, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds): SEER Cancer Statistics Review, 1975–2010, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. (2012) [Google Scholar]
- 72.Seli E and Tangir J: Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol, 17(3), 299–308 (2005) [DOI] [PubMed] [Google Scholar]
- 73.Sonoda Y, Abu-Rustum NR, Gemignani ML, Chi DS, Brown CL, Poynor EA and Barakat RR: A fertility- sparing alternative to radical hysterectomy: how many patients may be eligible? Gynecol Oncol, 95(3), 534–8 (2004) [DOI] [PubMed] [Google Scholar]
- 74.Chen VW WX, Andrews PA.: Cancer in North America.1990–1994: Volume one: incidence Sacramento. CA: North American Association of Central Cancer Registries. (1998) [Google Scholar]
- 75.Kim CH, Abu-Rustum NR, Chi DS, Gardner GJ, Leitao MM Jr., Carter J, Barakat RR and Sonoda Y: Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol Oncol, 125(3), 585–8 (2012) [DOI] [PubMed] [Google Scholar]
- 76.Plante M: Vaginal radical trachelectomy: an update. Gynecol Oncol, 111(2 Suppl), S105–10 (2008) [DOI] [PubMed] [Google Scholar]
- 77.Zuccotti M, Merico V, Cecconi S, Redi CA and Garagna S: What does it take to make a developmentally competent mammalian egg? Hum Reprod Update, 17(4), 525–40 (2011) [DOI] [PubMed] [Google Scholar]
- 78.Oktem O and Urman B: Understanding follicle growth in vivo. Hum Reprod, 25(12), 2944–54 (2010) [DOI] [PubMed] [Google Scholar]
- 79.Messinis IE, Messini CI and Dafopoulos K: The role of gonadotropins in the follicular phase. Ann N Y Acad Sci, 1205, 5–11 (2010) [DOI] [PubMed] [Google Scholar]
- 80.Gougeon A: Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod, 1(2), 81–7 (1986) [DOI] [PubMed] [Google Scholar]
- 81.Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S and Hardy K: Effects of follicle- stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod, 14(6), 1555–62 (1999) [DOI] [PubMed] [Google Scholar]
- 82.Louhio H, Hovatta O, Sjoberg J and Tuuri T: The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod, 6(8), 694–8 (2000) [DOI] [PubMed] [Google Scholar]
- 83.Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ and Hovatta O: Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab, 87(1), 316–21 (2002) [DOI] [PubMed] [Google Scholar]
- 84.Xu M, Kreeger PK, Shea LD and Woodruff TK: Tissue-engineered follicles produce live, fertile offspring. Tissue Eng, 12(10), 2739–46 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varghese AC, du Plessis SS, Falcone T and Agarwal A: Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol, 6, 47 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Telfer EE: In vitro models for oocyte development. Theriogenology, 49(2), 451–60 (1998) [DOI] [PubMed] [Google Scholar]
- 87.Gomes JE, Correia SC, Gouveia-Oliveira A, Cidadao AJ and Plancha CE: Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev, 54(2), 163–72 (1999) [DOI] [PubMed] [Google Scholar]
- 88.Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A and Falcone T: Three- dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol, 8, 119 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kreeger PK, Fernandes NN, Woodruff TK and Shea LD: Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod, 73(5), 942–50 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huntriss J and Picton HM: Epigenetic consequences of assisted reproduction and infertility on the human preimplantation embryo. Hum Fertil (Camb), 11(2), 85–94 (2008) [DOI] [PubMed] [Google Scholar]
- 91.Picton HM, Harris SE, Muruvi W and Chambers EL: The in vitro growth and maturation of follicles. Reproduction, 136(6), 703–15 (2008) [DOI] [PubMed] [Google Scholar]
- 92.Woodruff TK: The Oncofertility Consortium-- addressing fertility in young people with cancer. Nat Rev Clin Oncol, 7(8), 466–75 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD and Woodruff TK: In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod, 24(10), 2531–40 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]


