Abstract
This study experimentally assessed bacterial water-to-air partitioning coefficients resulting from showerhead aerosolization of water contaminated with Brevundimonas diminuta or Pseudomonas aeruginosa, and estimated human exposure through inhalation. Dechlorinated tap water was spiked with two cell densities (109 and 1010 CFU l−1) and cycled at three temperatures (10, 25, and 37 or 40ºC) through a full-scale shower system. For reproducibility, spiked water concentrations were intentionally higher than found in natural environments. Three types of samplers measured size distribution and viable concentrations throughout the system. Results indicate low levels of respirable bioaerosols were generated. The ratio of bacterial contaminant that was effectively aerosolized (bacterial water-to-air partitioning coefficient, PCbwa) was low – averaging 1.13×10−5 L m−3 for B. diminuta and 8.31×10−6 L m−3 for P. aeruginosa. However, the respirable fraction of aerosolized organisms was high, averaging above 94% (in shower) and above 99% (downstream) for both organisms. This study found no significant difference in bioaerosol load for a forward facing versus reverse facing individual. Further, for the average hot shower (33–43°C) the total number of respirable bioaerosols is higher, but the observed culturability of those aerosolized cells is lower when compared to lower temperatures. Bacterial water to air partitioning coefficients were calculated to predict microbial air concentration and these empirical parameters may be used for assessing inhalation as a route of exposure to pathogens in contaminated waters.
Keywords: Pathogen, Exposure assessment, Partitioning coefficient, Water infrastructure, Bioaerosols
INTRODUCTION
Respirable aerosols (i.e., those with diameters ≤10 μm) are generated during showering through several interdependent aerosol formation and removal mechanisms (Xu and Weisel 2003; Cowen and Ollison 2006; O’Toole et al. 2009). These aerosols may be inhaled by the shower occupant along with those in the same room or household. Researchers demonstrated via optical counting and sizing techniques that everyday showering generates 50% (mouth breathing) to greater than 86% (nose breathing) of the aerosolized particles deposited in the extrathoracic region, which may contain volatile and nonvolatile compounds (Xu and Weisel, 2003; Zhou et al. 2007; Hamilton and Haas, 2016). Though it can be conjectured that it is likely that less-volatile materials, such as microbes, could be aerosolized, the extent to which this is true has not been sufficiently studied or documented.
Researchers have studied to quantify the extent to which bioaerosols are generated from shower water contaminated with chemicals and microorganisms (Table 1). Particles around the size of a small bacterium (spherical, rod-shaped or filamentous bacteria: 0.25 to 5 μm) deposit in the human alveoli, while larger particles (spiral, cylindrical, or elongated >10 to 20 μm) deposit in the upper respiratory tract (Thomas et al. 2008; Ghosh et al., 2015). Several studies demonstrated that Legionella pneumophila (2 μm in length by 0.3–0.9 μm in width) can be aerosolized by showerheads (and hot water taps) during routine use, and that the aerosol particle size are small enough (<10 μm) to penetrate into the lower human respiratory system (Bollin et al. 1985; Deloge-Abarkan et al. 2007; Whiley et al., 2015). Though these studies showed very low numbers of culturable organisms, the authors indicate that these results confirm suspicions that bioaerosols containing L. pneumophila are created within showers, and thus may be a source of human infection when domestic water systems are contaminated (Dennis et al. 1984).
Table 1.
Test Conditions for Characterizing Aerosols during Past Showering Studies.
| Water Contaminant |
Cubicle Dimensions |
Water Temperature |
Probe Position |
Nozzle Location |
Monitoring Time | Measuring Technique |
Reference |
|---|---|---|---|---|---|---|---|
| Chloroform | 1.53 m3 1.53 (h) × 1.18 (w) × 0.85 (d) |
35–45 ˚C | Center of exhaust vent | Top center with water sprayed directly on floor (135 cm) | Every 2 min during 15 min | Hot wire anemometer (model AIMS droplet counter) |
Keating et al., 1997 |
| Heloacetic acids and haloketones | 6 m3 2.39 (h) × 2.67 (w) × 0.94 (d) |
36–38 ˚C | At breathing height 1.5 m through 1.75 m | Top of shower stall | Total 42 min with a 6 min background, 10 min shower on and 26 min shower off period | Optical particle counter (Lasair model 1002) | Xu and Weisel, 2003 |
| Salts | Full size tub within 11 m3 bathroom | Cold and Hot | Top of shower curtain (2 m) and 1 m above floor | Mannequin was positioned in shower spray cone to simulate splashing | 5–10 min before shower on, 10 min shower on and 10 min after shower off | Climet monitors | Cowen and Ollison, (2006) |
| Elemental constituents of water | 2.0 m3 2.41 (h) × 0.92 (w) × 0.92 (d) |
24–25 ˚C and 43-44 ˚C | Breathing height 1.5 m inside and outside shower | Water showered on mannequin at 30˚ angle | Aerodynamic particle sizer completed one measurement every min for each 10 min showering period. Next test period began when room temperature and humidity were back to baseline | Nephelometerbased DataRAM real time particle monitor and aerodynamic particle sizer (APS 3310) | Zhou et al., 2007 |
| City water with Mycobacterium mucogenicum and Pseudomonas aeruginosa | 9 m3 bathroom with 2.2 m3 curtain closing shower stall | 33–43 ˚C | 1.5 m from the floor | Four shower stalls during two seasonal sampling periods (two stalls per season) | Each shower stall was sampled for 3 days with a conventional showerhead in place and for 3 days with a membraneintegrated showerhead installed in the same shower. | Swirling aerosol collectors for 90 min | Perkins et al., 2009 |
| City water from UK with Legionella pneumophila |
99 showers from 82 households | 26.8 – 33 °C | 1 m from the shower at a height of 1.6 m | Plastic and metal | Sampled at the time of shower activation for a period of five and 10 min | six-stage viable Andersen sampler at a flow rate of 28.3 L/min. | Collins et al., 2017 |
Previous studies have proposed using an approach similar to the partitioning of volatile organic compounds to determine the bacterial aerosolization from a contaminated source (Angenent et al., 2005; Saini et al., 2011; Barnewall et al., 2015; Lee et al., 2016). Various researchers (European Commission, 2003; Bennett and Parks, 2006; Armstrong and Haas, 2007; Kulkarni et al., 2011; Moore et al., 2015) defined the bacterial water-to-air partitioning coefficient (PCbwa) as a ratio of the bacterial air concentration (CA, CFU m−3) to the initial source water concentration (CW, CFU L−1; Equation 1).
| 1 |
Thus, if the PCbwa is known, the exposure dose of bacteria may be quantified with only a few key parameters (such as measurements of the total volume of air respired and the concentration of airborne pathogen). Besides a lack of experimental data, the bacterial water-to-air partitioning coefficient assumed to vary significantly depending upon a number of factors, including several associated with the showerhead (e.g., make/model, operating pressure, liquid flow rate), the shower (e.g., geometry, presence of obstacles, sampling location, air exchange rate), characteristics of the contaminant (e.g., volatility, stability), and the amount of time needed for a sample to reach equilibrium (Bollin et al., 1985; Zhou et al. 2007; Perkins et al. 2009). The water-to-air partitioning coefficient of pathogens are dependent on additional properties including type and surface properties of pathogen (hydrophobic-hydrophilic interactions, surface charge), water quality (pH, alkalinity, suspended solids, organic carbon), and deposits on water distribution system (minerals and biofilm deposits) (Chattopadhyay and Puls, 1999; Chattopadhyay et al., 2002; Chattopadhyay et al., 2004; Chattopadhyay, 2016).
The utility of a partitioning coefficient for microorganisms has been established (Saini et al., 2011), yet there has been little experimental work to estimate this coefficient. The estimation of partitioning coefficient is proposed as a screening method to compensate for this lack of exposure data to assess the concentration of microorganisms in aerosols given their concentration in water. The objective of the present study was to perform exposure assessment of pathogen contaminated water during household activities under emergency situation. Bacterial water-toair partitioning coefficients resulting from showering with water contaminated with Brevundimonas diminuta or Pseudomonas aeruginosa were estimated to predict the fate of bioaerosol in case of large-scale accidental or intentional release. B. diminuta (length = 0.68 μm × width = 0.31 μm), was selected for these tests based upon its aerosolization potential due to its model representation of a water borne, gram negative organism, considered standard challenge organism for qualifying filters accepted bacteria of choice by (ASTM 2001), and can be consistently cultured under controlled conditions to yield very small, monodispersed cells with a narrow size distribution. P. aeruginosa (length = 0.5 μm to 0.8 μm × width = 1.5 μm to 3.0 μm) was selected based upon its known significance as a waterborne pathogen in the household installations and reservoirs, posing risks to immunocompromised individuals. For these tests, a controlled laboratory test system, including a full-size shower setup, adult-sized mannequin, and appropriate sampling equipment were designed and constructed.
MATERIALS AND METHODS
Experimental Design
Tap water, referenced as test water in this manuscript, spiked to two microbial concentrations (109 and 1010 CFU L−1) and water temperatures (10, 25, and 37 or 40ºC), in triplicate, flowed through a full-size shower system. Though B. diminuta tested for two concentrations at 10˚C, the test for P. aeruginosa at 10˚C was only performed at one concentration due to the available resources. Aerosol samplers were placed at three locations within the shower stall, and in the downstream section of the chamber (Figure 1). The temperatures were selected to represent conditions for cold, cool, and hot showers, respectively.
Figure 1. Schematic of the test chamber as configured to support shower tests.
Side and top views.
A wide variety of bioaerosol sampling methods are in use and numerous other methods are in the developmental stage. There is lack of standard protocols for aerosol sampling and sample preparation. Generally, the desired properties exist in the variety of aerosol samplers, but rarely in a single sampler (Grishpun et al., 2007; Ghosh et al., 2015). The time and space dependent characteristics in bioaerosol concentrations have great effect on determining the optimal sampling duration and location, while the overall performance of an aerosol sampler can be determined by a) physical factors (inlet sampling efficiency and collection efficiency) and b) biological factors (preserving biological characteristics of microorganisms during sampling and analysis). The number and location of sampling points were selected according to the variability of microorganism concentration, shower stall configuration, size of the concerned area, and the number, size, and relative proximity of emission sources. Laboratory comparison of the performance of bioaerosol samplers were performed using eight samplers (All Glass Impinger,AGI-30; SKC BioSampler; Wetted-Wall Cyclone Reference Sampler; gelatin filters; Multistage Liquid Impinger (MLI); Andersen Cascade Impactor (ACI); Cascade Impactor; and Electrical Low Pressure Impactor) were evaluated for their ability to collect bioaerosols generated in the laboratory setting (data not shown). Based on these tests (results not discussed here to maintain brevity), three sampling devices were shortlisted for use in the shower exposure tests: All-Glass Impinger (AGI) for measuring total bioaerosol concentration, and the Multi-stage Liquid Impinger (MLI) and the Andersen Cascade Impactor (ACI) for measuring the bioaerosol size distribution. Bioaerosol concentrations have considerable temporal and spatial variation because biological sources may not generate aerosols continuously (Table 2). For each subsequent test, eight glass impingement samplers (AGI-30, #7540, Ace Glass Inc., Vineland, New Jersey) and a single multi-stage liquid impinger (MLI, Copley Scientific Ltd., Nottingham, United Kingdom) collected samples from the breathing zone of an adult mannequin inside the shower. Forward and rear facing samples were collected based upon the fact that, during showering, most people spend a considerable portion of time facing both directions. The samples collected within the shower were taken to assess the individual exposure for a person showering, while downstream samples were collected to assess exposure to individuals outside the shower stall, but still within the room. Downstream samples were collected next to the exit plane with an AGI-30 and an Andersen Cascade Impactor (ACI, ThermoFisher Scientific, Franklin, Massachusetts). Two background samples were collected prior to each test using two AGI-30 samplers; one placed inside the shower and a second placed in the downstream sampling location. Background samples were collected for 15 min prior to each test before running the shower setup. All samples were analyzed via standard microbiological plate and count assays. The results from these samples were used to calculate several parameters, including bioaerosol concentration, respirable fraction, and PCbwa as a function of test water concentration and temperature (see Table 4). In total 12 AGI-30s, one MLI, and one ACI sampler were used for each test. The flow rate for each device was calibrated prior to testing using a DryCal® (Bios International; Butler, New Jersey) flow meter, and verified to be within ± 5% of the expected value. The standard flow rates for the AGI-30s, ACI and MLI were 12.5, 28.0 and 60.0 L min−1, respectively. The AGI-30s and MLI samplers were cleaned prior to each test by soaking in a 0.05% sodium hypochlorite solution followed by rinsing with 70% isopropyl alcohol and sterile distilled water; the AGI-30s were also autoclaved. The AGI-30s were filled with 20 mL PBS within a biosafety cabinet, and then transferred to the test chamber laboratory where they were connected in the appropriate positions within the shower test system. Similarly, 20 mL of PBS was added to each of the four stages of the MLI and a backup AGI-30 was connected to the exhaust of the MLI to act as a fifth stage capturing bioaerosol particles with diameters less than 1.7 μm (the lower size cut of the last stage in the MLI). A tee was placed in the line between the MLI and the AGI-30 to ensure proper flow rates within each sampler. Tryptic Soy Agar (TSA) plates were used in the ACI.
Table 2.
Comparison of Selected Bioaerosol Samplers.
| Device | Mechanism | Sampling Approach |
Viability | Advantage | Disadvantage |
|---|---|---|---|---|---|
| Cascade Impactor |
Sampling air stream makes a sharp bend and particles are stripped based on their aerodynamic diameter |
Provides the best size distribution information. 1 and 12 stages for aerosols with aerodynamic diameters from 10 nm to >18 μm | Only at 28 L/min collection rates and requires direct sampling onto agar plates | • Best ability to define particle size distributions • Models available to perform culturing |
• High cost per sampler, especially for high volume samplers • Sampling inefficiencies due to particle bounce • Not sensitive as total sampled mass is divided among multiple stages |
| Liquid Impingement |
Sampling air passes through a small opening and captured into a liquid medium | Efficiency drops in low volume glass impingers below aerodynamic diameters of 1 μm |
Impingers are flexible since pathogens are impinged into liquid media or buffer and can be used for culturing or molecular analysis | • Sample is collected into liquid and does not require extraction from a solid collection medium • Low cost of low flow glass impingers |
• Limited information on efficiencies, and the particle sizes • Glass impingers suffer from low sampling rate and limited sampling times due to evaporation |
Table 4.
Calculated Potential Exposure Dose and Bacterial Water-to-Air Partition Coefficient of Brevundimonas diminuta and Pseudomonas aeruginosa within the Shower and Downstream of the Shower.
| B. diminuta | P. aeruginosa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target Temperature | 10˚C | 25˚C | 40˚C | 10˚C** | 25˚C | 37˚C*** | ||||||
| CW, Tank Water (CFU L−1) | 1.01×109 | 1.01×1010 | 1.14×109 | 1.02×1010 | 5.86×108 | 6.74×109 | 1.26×109 | 1.02×109 | 1.24×1010 | 2.56×108 | 9.59×109 | |
| In Shower | MLI (CFU m−3) | 3.20×105 | 1.37×105 | 3.69×104 | 2.34×105 | 1.67×103 | 1.27×105 | 1.04×104 | 6.21×103 | 6.12×104 | 4.10×102 | 3.07×104 |
| % RF | 99.8 | 95.6 | 97.6 | 96.7 | 81.4 | 98.1 | 96.8 | 96.5 | 97.3 | 99.7 | 96.3 | |
| CA, AGI-30 (CFU m−3) | 1.78×104 | 9.25×104 | 1.86×104 | 1.01×105 | 5.66×103 | 3.49×104 | 9.51×103 | 7.36×103 | 6.57×104 | 4.32×103 | 4.37×104 | |
| Dexp (CFU) | 3.46×103 | 1.72×104 | 3.55×103 | 1.89×104 | 8.99×102 | 6.68×103 | 1.79×103 | 1.38×103 | 1.25×104 | 8.39×102 | 8.21×103 | |
| PCbwa (L m-3) | 1.76×10−5 | 9.19×10−6 | 1.64×10−5 | 9.85×10−6 | 9.66×10−6 | 5.18×10−6 | 7.57×10−6 | 7.25×10−6 | 5.29×10−6 | 1.69×10−5 | 4.56×10−6 | |
| Downstream | ACI (CFU m−3) | 3.96×104 | 1.31×105 | 2.54×104 | 9.11×104 | 6.89×103 | 5.64×104 | 4.39×103 | 1.01×104 | 1.33×104 | 1.79×102 | 1.79×102 |
| % RF | 99.8 | 99.5 | 99.7 | 99.5 | 99.6 | 99.5 | 100.0 | 100.0 | 100.0 | 99.9 | 100.0 | |
| CA, AGI-30 (CFU m−3) | 5.22×103 | 2.77×104 | 3.76×103 | 2.85×104 | 7.07×102 | 5.02×103 | 6.76×101 | BDL | 5.39×102 | BDL | 2.20×102 | |
| Dexp (CFU) | 1.02×103 | 5.38×103 | 7.31×102 | 5.53×103 | 1.37×102 | 9.75×102 | 1.32×101 | 1.96×103* | 1.05×102 | 3.48×101* | 4.28×101 | |
| PCbwa (L m-3) | 5.18×10−6 | 2.75×10−6 | 3.30×10−6 | 2.80×10−6 | 1.20×10−6 | 7.45×10−7 | 5.38×10−8 | 0.00×100 | 4.34×10−8 | BDL | 2.29−10−8 | |
CW, Concentration of the source water; MLI, concentration of viable aerosols collected with the MLI in the shower; ACI, concentration of viable aerosols collected with the ACI downstream of the shower; %RF, Percent respirable fraction of the MLI or ACI data; CA, Viable aerosol concentration as calculated with the AGI-30 (sampler); Dexp, Calculated Potential Exposure Dose for the 15 min exposure duration; PCbaw, bacterial water-to-air partition coefficient. A breathing rate of 13 L min−1 was assumed in determining the potential exposure dose. For all data points n=3. BDL - Below Detection Limit (<0.33 CFU m−3).
Exposure doses were calculated using ACI (sampler) concentrations rather than AGI total concentrations.
Test performed at one concentration.
Appreciable die-off of the cells (approximately two orders of magnitude reduction) in the source water tank and elimination of viable P. aeruginosa (at a concentration of 109 CFU L−1) after passing through the showerhead during the tests run at the 40˚C suggested that P. aeruginosa was not viable at water temperatures at or above 40˚C. Thus, the high temperature testing at 1010 CFU L−1 was conducted at 37˚C.
Testing Chamber and Shower Apparatus
The shower tests were conducted in a relatively large-scale aerosol chamber designed and constructed to provide a facility for bioaerosol testing and sampling under defined, repeatable, controlled conditions. The test chamber was operated at its intended flow setting of 252 m3 hr−1 corresponding to approximately 15 air changes per hour for all tests, which is within the recommended range typical for household bathrooms (ASHRAE 2004). A residential full-size bathtub/shower stall was installed in the test chamber for the shower tests (Figure 2). Duplicate 120 gallon storage tanks were used for test water storage and waste water collection. A centrifugal pump (Dayton, Model 4TA97) was used to transfer the test water from the storage tank to the shower stall and a residential sump pump was used to transfer the captured waste water from the shower drain to the waste water storage tank. One inch outer diameter crosslinked polyethylene tubing (Zurn Pex, Inc., Commerce, Texas) was used for all the plumbing associated with the recirculation of the test water, and the plumbing to and from the shower. Soft plastic tubing was used for drainage from the shower stall to a capture basin containing the system sump pump. An in-line flow meter (King Instruments 0 to 5 gal min−1, Garden Grove, California) and a pressure gauge (Ashcroft 0–30 psi, Stratford, Connecticut) were installed in the source line between the source pump and the showerhead to monitor the water flow rate and pressure during testing. The showerhead was installed in the bath/shower unit, and an adult-sized mannequin was used as a surrogate shower occupant. A sampling valve and line was installed immediately upstream of the showerhead to allow for the collection of water samples during the shower test runs.
Figure 2.
General configuration of test chamber and air flow path.
System Characterization
Prior to conducting showering testing with microorganism suspensions, the test system was characterized to ensure proper operation and to determine the aerosol formation rates from tap water under the various testing conditions to be evaluated with the bacterial suspensions. The continuous particle size distributions were measured to assess the degree of impact various parameters including a range of showering conditions [room temperature (25 °C), shower duration (15 and 30 minutes), different types of nozzles and showerheads (Waterpic Model SM651; Plumb Shop/PS2; Peerless Power Spray Model # 76151; and Delta Faucet 52650-PK), water flowrate (3–4 L/min and 5–6 L/min), pH (4, 7, and 10), relative humidity (95%), mannequin position, and the effect of dissolved solids (360 mg/L MgSO4) in the water on aerosol formation rates]. Four showerheads were used to characterize the aerosol size distributions during the system characterization and the nozzle (Plumb Shop/PS2, Novi, Michigan) that produced the greatest concentration of respirable particles was selected as the nozzle for subsequent testing. The configuration of the selected nozzle for the test runs consisted of a ring of ten apertures, which produced a high pressure spray. The other showerheads for initial characterization study had up to 127 apertures that produced much gentler sprays. During the test runs, the air exchange rate in the test chamber was determined by releasing a small amount of sulfur hexafluoride (SF6), an inert tracer gas, into the room and collecting air samples at several times during the run. The samples were subsequently analyzed for SF6 by gas chromatography and the air exchange rate was determined by fitting a first-order decay equation to the SF6 data.
Preparation and Characterization of Vegetative Cells
A working freezer stock for both B. diminuta (ATCC #11568; Lot No. BD01; frozen in glycerol) and P. aeruginosa (ATCC #27853; Lot No. PA01; frozen in glycerol) were generated specifically for this study and were used for all sampler characterizations and shower tests. The cell banks of B. diminuta and P. aeruginosa were determined to have a concentration of 1.1×1011 and 7.0×1010 CFU mL−1, respectively. Each freezer stock was characterized for purity through observed colony morphology, wet mounts, and gram stains of cells grown on TSA plates. Working cell stock concentrations were determined by enumerating three random aliquots taken from freezer stock using standard microbiological spread plating using TSA and phosphate buffer saline (PBS) diluent.
Water Storage Tank Preparation
Prior to each test, the storage tank was filled with approximately 150 L of tap water (Columbus Water Treatment Plant, Dublin Road, Ohio). The average alkalinity (U.S. EPA Method 310.1/2320B), turbidity (Method 180.1), free and total chlorine (HACH® Methods 10245 and 10250), total organic carbon (TOC) (Method 5310C) concentrations in the tap water before autoclaving were 54 mg/L as CaCO3, 0.07 nephelometric turbidity units (NTU), 1.55 mg/L, and 3.02 mg/L, respectively. The water circulating the shower prior to spiking system was sterilized by autoclaving. The integrator strips monitor was used to verify effectiveness of water sterilization. The pH was measured using Corning® pH/ion meter model 450. The pH of the tap water was 7.4. Once in the tank, the target temperature was achieved by either raising the temperature using a heat tape affixed to the water recirculation loop, or cooled by flowing liquid nitrogen through a large copper coil submerged in the storage tank. Upon reaching and maintaining the desired temperature, the free chlorine concentration in the tap water was measured using a HACH® Chlorine Test kit (N, N′-diethyl-p-phenylenediamine, DPD, Free Chlorine reagent, Loveland, Colorado). The tap water was dechlorinated with 75 mL aliquots of 10% sodium thiosulfate until the free chlorine concentration in the tank measured ≤0.02 mg/L. The appropriate liquid suspension of cells was then added and mixed via the recirculation pump for at least 10 min prior to starting the test. Immediately before and after each 15 min test, a liquid sample was withdrawn from the storage tank to measure the initial and final bacteria concentrations in test water. Water samples were also collected before and after the showerhead to account of bacterial losses within the tubing and showerhead. The showerheads were cleaned and disinfected in between the runs by soaking in >0.5% sodium hypochlorite for approximately 10 minutes, rinsing with distilled water and 70% isopropanol and allowed to dry.
Test Procedures
For background sampling, one AGI-30 was placed inside the shower and a second was placed in the downstream sampling location. The background samplers were run for 15 min prior to each test. Sample probes were passed through the shower wall and through the test chamber wall, such that seven of the AGI-30 samplers were located outside of the chamber (three in one probe, four in the other, Figure 1). The sample probes extend horizontally outward to the breathing zone of the mannequin, and allowed easy access to the samplers throughout the study. However, because of the high MLI flow rate and the design of the probes, the MLI could not be used within either probe without adversely affecting the flows of other samplers using the same probe. Consequently, the MLI was installed and operated inside the shower to limit potential interference with other samplers. An eighth AGI-30 was also located inside the shower and used to assess for sample loss in the sampling probes using statistical analysis between the various AGI-30 locations. Both the MLI and the eighth AGI-30 were located near the probe located behind the mannequin to avoid contamination from splashing shower water. A final AGI-30 and the ACI were located inside the exit duct of the chamber, upstream of the exhaust filter.
After retrieval of the background samples, the valve from the storage tank to the showerhead was opened and all bioaerosol samplers, with the exception of the ACI, were turned on and their proper function visually verified. Since the air sampled by the ACI impacts directly onto growth media, the sampling duration for this sampler needed to be varied as a function of the test water contamination level to avoid overloading the collection plates. Therefore, ACI samples were collected for 1 min at 109 CFU L−1 and 10 sec at 1010 CFU L−1, 5 min into each test. Two to three min into the shower test, a liquid sample of approximately 25 mL was retrieved directly from the shower line via the bypass valve located outside the test chamber. The shower supply liquid flow rate and pressure were monitored throughout the test to verify that the shower was functioning as desired (6.0 ± 0.6 L min−1 and 35 psig, respectively, for all tests). Fifteen min after being started, the water supply to the shower and the vacuum pumps to all the bioaerosol samplers were simultaneously shut down to conclude the showering event. Between trials, the shower stall walls were rinsed with distilled water and dried.
Sample Processing and Enumeration
After each completed test, the AGI-30 and MLI samplers were disconnected from the test system, all open connections were wrapped in Parafilm™ (Bemis NA, Neenah, Wisconsin) and the samplers were transported to the analytical laboratory for processing. The contents of the AGI-30 samplers were transferred to sterile 50 ml tubes to measure and record the total sample volume before enumeration. Samples were collected from each stage of the MLI, and their volumes were recorded. A 20 mL PBS rinsate was also collected from each MLI stage and analyzed separately. Aliquots of all liquid samples, including samples collected from the storage water tank and the showerhead, were vortexed before enumeration. All liquid samples were enumerated following standard microbiology plate count methods (Clescerl et al. 1998).Triplicate 100 μL aliquots of three 10-fold serial dilutions of the liquid samples were plated onto TSA and were incubated at 37°C until 25–250 colonies grew to a distinguishable size (18–24 hrs). Likewise, the agar collection plates in the ACI were removed from the sampler and transported to the analytical laboratory for incubation and enumeration. For the liquid samples, the total number of cells recovered in each sample was calculated based on the volume of the sample and the resulting CFU concentration.
DATA ANALYSIS
Bioaerosol Concentration
The bioassay data from the AGI-30 and MLI samples establish a value of CFU per mL of sampler fluid. These results were converted to bioaerosol concentrations (Equation 2).
| 2 |
Where CA is the total bacterial air concentration (CFU m−3), CL is the CFU mL−1 of sampler fluid cultured, VL is the total volume of liquid in the sampler in mL, sampler rate is m3 min−1, and T is sampling time (min). The conversion determines a total bioaerosol concentration for the AGI-30 and stage-specific bioaerosol concentrations for the MLI. The raw CFU plate−1 results from the ACI were converted to stage specific bioaerosol concentrations (Equation 3).
| 3 |
Where CPlate is the counted CFU plate−1 results and T is the sampling time (min). For each sampler the appropriate air sampling flow rate was employed (12.5, 28.0, or 60.0 L min−1 for the AGI-30, ACI and MLI, respectively). Unit conversions of operating parameters were performed during calculations as appropriate.
Statistical Analysis
An analysis of variance (ANOVA) test was used to detect if the measured bioaerosol concentrations generated from different sampling locations or different testing conditions (i.e., water temperature, initial cell inoculum concentration) were statistically different from one another. Results were considered significant if p < 0.05.
Particle Size Distribution
The bioaerosol particle size distribution was calculated from the culturable analysis of the collected material on each size-dependent stage of the MLI and the ACI. The ACI is a six-stage viable particle sampler that was used to determine particle size distribution and concentration of collected culturable bioaerosol fractions. During operation, the aerosol particles were impacted onto one of the six stages, depending on the size of the particle. The particle size cut-points for the ACI are 7, 4.7, 3.3, 2.1, 1.1, and 0.65 μm. The MLI is a five-stage, size selective impinger. The MLI cut-points are nominally 13, 6.8, 3.1, 1.7 and <1.7 μm. The air sample is collected on sintered glass disks for each of the first four cutpoints that were continually wetted by the collection liquid. For this testing, a portion of the exhaust was subsequently collected in an AGI30 to capture bioaerosols below 1.7 μm. Because of the design of the two samplers, the results from the MLI and the ACI are treated differently. It is possible that clusters of cells behaving as a single aerosol particle were broken apart in solution during the sample processing of the MLI and were subsequently counted as more than a single CFU. Conversely, the ACI particles impact directly on the growth media and a cluster of multiple cells would result in a single CFU during counting. The data from the MLI are reported in terms of a mass median aerodynamic diameter (MMAD), whereas the data from the ACI are reported in terms of a number median aerodynamic diameter (NMAD) to better account for the inherent differences in their sampling mechanisms. The MMAD and NMAD are defined as the diameter at which 50% of the mass (here, it is assumed that each CFU has equivalent mass) or number of the collected bioaerosols, respectively, lies both above and below that size. The geometric standard deviation (GSD) provides an indication of the width of a size distribution (or degree of polydispersity) - the larger the value the greater is the spread in the size distribution (Thorpe, 2007; Allegra et al., 2016). The GSD is defined as the ratio of the diameter at which 84% (i.e., one standard deviation above the mean) of the mass lies below that size and the MMAD or NMAD (Peters et al., 2001; Byers et al., 2013). The MMAD for the MLI data, the NMAD for the ACI data, and GSD for both data sets were determined by calculating the cumulative percent of mass less than that found on the preceding stage:
| 4 |
where x is the number of stages in the sampler. Using a logarithmic trend line, the respirable fraction (RF) of viable bioaerosols (viable cells with an aerodynamic diameter ≤10μm are relevant for lower respiratory system) were determined. Using the RF determined from the MLI and the ACI, the respirable concentration of viable bacteria collected through the multiple AGI30s within the shower and downstream of the shower were determined.
Respirable Fraction and Dose Calculation
From the size distribution data collected by the MLI and ACI, the fraction of the distribution that lies within the respirable size range was calculated as the RF.
| 5 |
For simplicity, it was assumed that all available respirable aerosols (≤10 μm aerodynamic diameter) were inhaled and retained, i.e., the human retention rate (RR) was assumed to be 100% for total respiratory track deposition (USEPA 2004). Using an exposure dose model proposed by EPA (USEPA 2011) and used by researchers (Bennett and Parks, 2006; Armstrong and Haas 2007; Moore et al. 2015) for an inhalational dose of Bacillus atrophaeus, Pseudomonas aeruginosa, MS-2, and Legionella in a spa setting, an exposure dose for a non-volatile agent within a single shower can be estimated (Equation 6).
| 6 |
The calculated bioaerosol concentration (CA, CFU m−3) and respirable fraction (RF) along with an assumed inhalation rate (I) of 13 L min−1 (USEPA 2011) were used to calculate the potential exposure dose (Dexp, CFU) of respirable particles for a 15 min duration of exposure (T) under each tested shower condition.
RESULTS
Aerosol Generation Characterization
An assessment was conducted to ensure there was a homogenous concentration of shower aerosol within the chamber. Aerosol concentrations were measured in parallel planes perpendicular to the plane of the shower curtain using an optical particle counter/sizer (Climet CI-500, Redland, CA). The aerosol measurements were made in three planes that were located: (a) near the front of the shower, (b) just in front of the mannequin, and (c) behind the mannequin near the back of the shower. Relative standard deviations of the nine measurements made in each of three planes varied from 6% to 17% suggesting that there were no large variations in the aerosol concentration in the three planes. Likewise, the differences in the average aerosol concentrations measured for the three planes were between 5% and 12% indicating that there were no large variations in the aerosol concentration in front of and behind the mannequin. This fact suggested that the aerosol inhalation exposure was the same regardless of a person’s orientation in the shower. The total aerosol produced from the unspiked test water was monitored both within and downstream of the chamber. During this test, the aerosol concentration in the downstream section was lower than in the shower for the majority of the test period (Figure 3), and there was an elapsed time for the aerosols to travel between the two sampling locations. Measurements were used to calculate the standard time constant of first order varying systems (time to reach 63% of the max concentration) for an aerosol generated in the shower at both the breathing height and in the downstream section. The time constant was approximately two min for inside the shower, and 3.5 min for the downstream section. The rise time (time to rise from 10% to 90% of the max concentration) was 4.5 min for inside the shower, and 7.7 min for the downstream sampling location.
Figure 3.
Time Constant Data for Tap Water Aerosol Generated in the Shower.
Test Water Microbial Concentrations
Samples of the test water were collected before and after each test to determine the initial microbial concentration, and to establish if a loss of viable microorganisms occurred throughout the tests. In general, the desired test water concentrations were achieved, and only a slight loss occurred during transport to the shower system; the total loss of viable cells between the pre- and post-test test water concentrations was on average less than one order of magnitude (Table 3). However, there was appreciable die-off of the P. aeruginosa cells (approximately two orders of magnitude reduction) in the storage tank after passing through the showerhead during tests run at the 40˚C target temperature for the 109 CFU L−1 concentration.
Table 3. Average Pathogen Concentrations in Water.
The average concentrations measured at each target concentration for each type of sample across all temperatures (std dev) for the test water through the plumbing used in the analysis. Averaged results are given in CFU L−1 for Brevundimonas diminuta and Pseudomonas aeruginosa tests at each target test water concentration.
| Water Sample | Water Concentration (CFU L−1) |
|||
|---|---|---|---|---|
| B. diminuta | P. aeruginosa | |||
| 109 n=9 |
1010 n=9 |
109 n=7 |
1010 n=6 |
|
| Pre-Test Tank Water | 1.1×109 (7.6×108) | 1.0×1010 (3.4×109) | 1.6×109 (9.4×108) | 1.4×1010 (6.7×109) |
| Pre-Showerhead | 8.6×108 (3.9×108) | 9.1×109 (1.6×109) | 8.8×108 (5.0×108) | 8.9×109 (3.9×109) |
| In Shower | 8.6×108 (2.2×108) | 8.0×109 (1.7×109) | 8.1×108 (5.8×108) | 1.0×1010 (2.4×109) |
| Post-Test Tank Water | 8.5×108 (2.6×108) | 8.9×109 (2.7×109) | 7.9×108 (5.1×108) | 1.2×1010 (2.6×109) |
Aerosol Concentrations
The viable bioaerosol concentrations (CFU m−3) measured using AGI-30s within the shower agreed well with one another (Figure 4). A multi-factorial ANOVA concluded that there was no statistical difference (p=0.096) in the samples collected from the three different sampling locations within the shower for B. diminuta. Furthermore, the in-shower B. diminuta samples indicate that the bioaerosol concentrations at 10 and 25˚C were not significantly different (p=0.75), but the bioaerosol concentrations at 40˚C were statistically lower for both initial inoculum concentrations (p<0.01; Figure 4A). The analysis of the liquid samples collected during the test show little cell die-off in the storage tank, in the plumbing, or after exiting the showerhead (Table 3), indicating that the cells are stable at temperatures above 25˚C and that forces introduced while passing through the showerhead did not reduce viability. For P. aeruginosa, the results were similar, but are more limited due a lower number of conducted trials. The number of viable aerosolized P. aeruginosa cells collected within the shower suggests that there was no difference in the three in-shower sampling locations, but that there was a strong dependence on temperature (Figure 4B). The data for the P. aeruginosa in-shower bioaerosol concentrations at 10 and 25˚C were not significantly different (p=0.21), however the bioaerosol concentrations at 37˚C were significantly lower for both inoculum concentrations (p=0.048). This finding is consistent with the decreased viability of P. aeruginosa cells collected in the source waters at increased temperatures (Figure 4B, Table 3).
Figure 4. Average AGI-30 Bioaerosol Concentrations as Measured at Each Location within the Shower Setup: A- B. diminuta Results, B- P. aeruginosa Results.
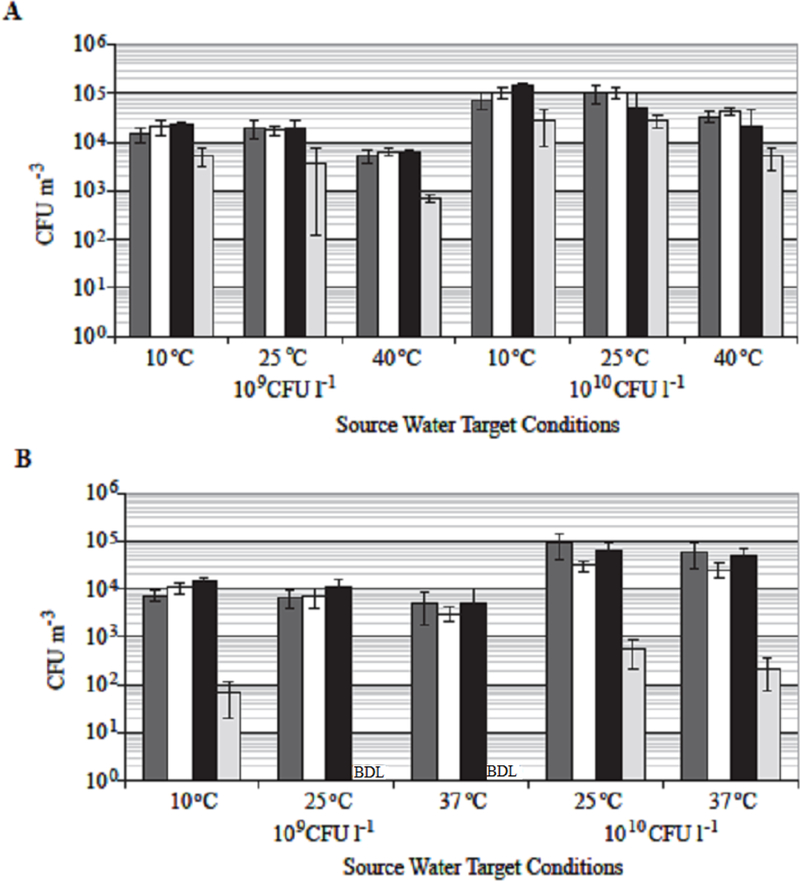
The error bars show the standard deviation of the results at each sampling location from the test runs conducted for each test condition. Below Detection Limit (BDL) - results were below the detection limit for the AGI-30 sampler. Coloring indicates sampler locations. Dark Gray: samplers collected through the probes In Front of the mannequin (n=4). White: samplers collected through the probes Behind the mannequin (n=3). Black: sampler located behind the mannequin Inside the shower stall (n=1). Light Gray: sampler located Downstream of the shower stall (n=1).
In each trial there was a decrease in the downstream bioaerosol concentrations. The B. diminuta downstream concentration was approximately 20% of the in-shower concentration (Figure 4A). As for the P. aeruginosa, there were multiple trials where no viable P. aeruginosa bioaerosols were recovered from the downstream AGI-30. Those tests where there was recovery of viable bioaerosols; the concentrations were very low and not sufficient to allow statistical quantification (Figure 4B). The average non-zero results show that viable aerosolized P. aeruginosa concentrations at the downstream location were approximately 1% of the in-shower concentration.
Particle Size Distributions and Respirable Fractions
Particle size distributions were determined both in the shower and at the downstream sampling location using the MLI and ACI samplers, respectively. The ACI sampler is designed to collect particles directly on growth medium; therefore, the sampling times used for the ACI must be carefully chosen to allow for the collection of sufficient particles for accurate quantification without overloading the collection plates. Since the ACI collection time was only a small fraction of the test period, they represent a snapshot of the downstream particle size distribution. The recovery results for the downstream samples were measurable, albeit quite variable and frequently below statistically meaningful levels. For the purposes of this report, only those results that had a peak concentration exceeding 30 CFU plate−1 are presented (Table 4).
Nominally, the measured MMADs and NMADs calculated from the MLI and ACI data averaged 2.6 μm and 4.3μm for B. diminuta and 3.9μm and 2.7μm for P. aeruginosa, respectively. Within the shower 50% of the viable cells aerosolized from the B. diminuta spiked shower water were less than 2.6μm in size and are well within the respirable range. These values were determined from probability plots of the cumulative mass fraction of cells versus their aerodynamic diameter. Similarly, the GSD values averaged 2.3μm and 1.3μm for B. diminuta within the shower and downstream of the shower, respectively. The GSD for P. aeruginosa averaged 1.6μm both within and downstream of the shower. Finally, the respirable fraction of viable cells was determined for each tested condition. The calculated RF from all tests averaged 99.6% of the total bioaerosol concentration for B. diminuta, and 99.9% of the total bioaerosol concentration for P. aeruginosa (Table 4).
Bacterial Water-to-Air Partitioning Coefficient
Bacterial water-to-air partitioning coefficients, PCbwa, were calculated from both the in-shower MLI sample results and the downstream ACI results for each temperature and initial test water concentration using Equation 1 (Table 4). The results illustrate that only a very small fraction of the B. diminuta or P. aeruginosa vegetative cells were effectively aerosolized and collected as viable bioaerosols during showering under the testing conditions in this study. The average PCbwa within the shower was 1.13×10−5 L m−3 for B. diminuta and 8.31×10−6 L m−3 for P. aeruginosa. Within the source concentration tested, the PCbwa values for B. diminuta and P. aeruginosa at a particular temperature varied and remained within a factor of two (downstream results for P. aeruginosa are the exception to this observation). In an effort to further substantiate the use of a water-to-air partitioning coefficient, the respirable aerosol concentrations within the shower were plotted against the initial test water concentrations (Figure 5). As indicated per Equation 1 and 2, linear trend lines were fit to the in-shower samples plotting the respirable concentration vs. water concentration. The squared correlation coefficient, R2, values for the linear-fit curves were within an acceptable range (0.82 for B. diminuta and 0.88 for P. aeruginosa). However, fitting the data to power functions fit the data more accurately (R2 = 0.84 and 0.91 for B. diminuta and P. aeruginosa, respectively) and is not impeded by an artificial intercept (at a source concentration of 0 CFU L−1 a linear approximation would result in an aerosol concentration of 5700 and 3400 CFU m−3 for B. diminuta and P. aeruginosa, respectively). Therefore, this data set implies that the bacterial water-to-air partitioning coefficient does not change linearly with increasing contamination levels.Unfortunately, due to the limited range of initial test water concentrations that were successfully tested, a complete characterization of bioaerosol partitioning coefficients, as a function of initial water concentration, is not possible.
Figure 5. Concentration of respirable Brevundimonas diminuta (Bd) and Pseudomonas aeruginosa (Pa) aerosols in the shower versus the initial test water concentration.
Inhalation Exposure Assessment
The inhalation exposure was calculated based on the bioaerosol data obtained from the AGI-30 results for each of the initial inoculum concentrations used during the simulated test conditions assuming a breathing rate, an exposure time, and the respirable fraction as calculated through the MMAD within the shower or NMAD downstream of the shower (Table 4). For simplicity it was assumed that 100% of the respirable bioaerosols were retained (Lindsley et al., 2017). Using these assumptions the calculated potential respirable dose for B. diminuta in a cool (25˚C) shower with a source water concentration of 109 CFU L−1 is 1.1×104 CFU from within the shower and 3.1×103 CFU downstream of the shower. For P. aeruginosa the estimated respirable dose for a cool (25˚C) shower with a source water concentration of 109 CFU L−1 P. aeruginosa is 6.9×103 CFU within the shower stall (Table 4). The downstream concentrations of viable P. aeruginosa bioaerosol were very low based on the samples obtained using the AGI-30 sampler, although the results of the ACI sampler suggest that a much greater concentration of viable bioaerosols were present downstream. For example, the average result collected above the limit of detection (>0.1 CFU mL−1 sampling fluid) from the AGI-30 for the 109 CFU L−1 indicate a downstream bioaerosol concentration of <0.01 CFU m−3, whereas the above the detection limit (>30 CFU plate−1) results from the ACI sampler indicate that the bioaerosol concentration was approximately 1.96×103 CFU m−3 (Table 4). Since the ACI samples are collected for only a short period of time during each shower test run, the measured bioaerosol concentrations were likely not equal to the average concentration during the 15 min shower, but are expected to be within 50% of the average concentration and are used here as an approximation of the average downstream concentration. The differences between the recoveries of viable bioaerosol with the AGI-30 and ACI may indicate that the collection process employed in the AGI-30 negatively impacts the survival of P. aeruginosa cells. Evidence of this phenomenon was observed in the sampler characterization testing (data not shown).
DISCUSSION
The objective of this study was to identify a bacterial water to air partitioning coefficient for P. aeruginosa and B. diminuta in a standard shower setup. The partitioning coefficient is dependent on the configuration/design of the aerosol system and its operational parameters and the species or strain of the agent (bacteria, spores, virus, or toxin) being aerosolized (Roy and Pitt, 2006; Barnewall et al., 2015). Change to the aerosol generation system and the operational parameters such as system flow rates or the type of aerosolized agent could impact the partitioning coefficient. Though this value is specific to the testing conditions, it provides a better estimate of microbial exposure within a shower than previously available in the literature (Table 5).
Table 5.
PCbwa values of Pathogens and other Microorganisms Released during Various Activities.
| Microorganism | Source of Aerosol | PCbwa (L m−3) | Rationale | Data Source |
|---|---|---|---|---|
| Total coliforms | Wastewater- irrigated fields |
6.9 × 10−6 | Outdoor study quantifying the number of viable coliform generated by sprinklers with discharges of 1.7, 4.5, and 100 m3 h−1 |
Teltsch et al. 1980 |
| Legionella pneumophila | Hot water (41°C) faucet for 7 min of flushing | 3.0 × 10−5 | Used filters and fluorescent in situ hybridization (FISH) | Deloge-Abarkan et al. 2007 |
| Endotoxin | Indoor swimming pool (40°C) |
Mean 2.3 × 10−5 (90% range 1.6 to 3.1 × 10−5) |
Monte Carlo simulation | Armstrong and Haas 2007 |
| Endotoxin of gramnegative organisms | Indoor swimming pool | 2.1 × 10−5 | Normal distribution of data | Rose et al., 1998 |
| Mesophilic bacteria TSA-SB bacteria* | Aeration of wastewater from two treatment plants | 9.6 × 10−7 | Wastewater temperature was 18˚C, 30 min aeration (rate of 16m3 hr1) followed by 1 hr nonaeration periods | Bauer et al. 2002 |
| P. aeruginosa (P. fluorescens, P. antarctica, and Brevundimonas vesicularis were also present in the water) | Spa pool water (35.2°C±3.1°C) |
<3 × 10−6 | Spa pool water was not changed/ disinfected, nor were the surfaces of the pool cleaned during 2month study. | Moore et al. 2015 |
|
Bacillus anthracis
Sterne (34F2) Yersinia pestis KIM5 strain Mycobacterium tuberculosis |
Sterile distilled water in aerosol chamber |
2.13 × 10−4 9.3 × 10−6 1.49 × 10−5 |
6-jet collision nebulizer operated at 13±1 L min−1 and 19±1 psi. For M. tuberculosis nebulizer operated at 19±1 L min−1 and 35±1 psi | Saini et al. 2011 |
| B. anthracis Ames | Sterile water | 2.30 × 10−7 - 7.11 × 10−7 | Three jet Collison nebulizer. Humidity = 53–83% |
Barnewall et al., 2012 |
|
Burkholderia pseudomallei strains 1026B, K96243, HBPUB10134A, and HBPUB10303A |
Aerosol generated from liquid suspension |
4.60 × 10−7 - 1.22 × 10−6 3.27 × 10−7 - 1.91 × 10−6 7.01 × 10−7 - 1.59 × 10−6 6.39 × 10−7 - 1.21 × 10−6 |
Three-jet Collison nebulizer. Humidity = 60.5–79.3%; temperature = 18.5 – 23.7˚C. | Barnewall et al., 2015 |
|
B. diminuta P. aeruginosa |
Full scale showering using city water | 5.18×10−6 - 1.76×10−5(in shower); 7.45×10−7 - 5.18×10−6 (downstream) 4.56×10−6 - 1.69×10−5 (in shower); BDL – 5.38×10−8 (downstream) |
15 min shower at different temperatures | Present study |
bacteria associated with certain waterborne virulence factors.
Bacterial Water to Air Partitioning Coefficient
The PCbwa values for vegetative B. diminuta and P. aeruginosa from this shower study (Table 4). As the bacteria concentration in the source water increased, the bacterial air concentration also increased. However, the change in bacterial air concentration with increase in water concentration appears to be non-linear. This type of non-linearity might be due to the aggregation of the pathogens in the water and/or air system. Change in partitioning due to aggregation was reported at higher concentrations of microorganisms in water and solid systems (Chattopadhyay and Puls, 2000; Faulkner et al., 2003). The PCbwa values for vegetative B. diminuta and P. aeruginosa via showering are comparable to the calculated PCbwa values of other types of bioaerosols generated in indoor-outdoor environments (Table 5). Table 5 lists the estimated PCbwa values based on release of pathogens and other microorganisms during various activities. Though each of the previous studies assessed drastically different sources and utilized variable quantification methods, the tabulated PCbwa values were relatively similar when compared to partitioning coefficients for similar species. Researchers have performed sampling and analysis to estimate release of chemical and radiological constituents and their impact during inhalation and dermal uptake (McKonel and Howd, 1992; Fitzgerald et al., 1997; Gordon et al., 2006; Weschler and Nazaroff, 2012). Typical water-to-air partitioning coefficients found for disinfection byproducts (DBPs) measured in chlorinated water aerosols are 0.02 to 0.2 L m−3 (Xu 2002; Xu and Weisel 2003). Clearly, this study demonstrates that there is a significant disparity between partitioning coefficients for DBPs and biological constituents within shower waters, and chemical partitioning coefficients should not be equivalently used for bioaerosol applications.
Inhalation Risk
Comparison of inhalation risk at the tested source water concentrations of B. diminuta and P. aeruginosa were higher than aerosol studies conducted at actual sites. The present study used 109 and 1010 CFU L−1 test water concentrations of the two test organisms. These cell concentrations were not selected to specifically mimic actual sites, but rather were used to ensure reliable detection of aerosolized organisms under worst-case situations. These initial concentrations are comparable to the concentrations of total bacteria found within wastewater treatment plants (Teltsch et al. 1980; Bauer et al. 2002). The average estimated exposure doses for B. diminuta and P. aeruginosa were 2.0×103 CFU and 1.3×104 CFU, respectively for the tested 15 min shower duration (or 132 CFU min−1 and 847 CFU min−1). These doses are higher than the estimated exposure dose within a hospital shower (0.4 CFU min−1) where the average culturable bacterial concentration in the source water was 7.6×103 CFU L−1 (Perkins et al. 2009).Likewise, the estimated exposure dose to Legionella pneumophila, which could be detected as a viable but non culturable (VBNC) state after disinfection (Leclerc, 2003; Li et al., 2014), in two French showers was 0.2 cells min−1 with an average water concentration of 4.6×105 cells L−1 (Deloge-Abarkan et al. 2007).
Location to the shower can influence the estimated exposure dose.
Crimi et al. (2006) measured Legionella pneumophila contamination in burn care bathtub and the air surrounding the water tap at various distances. Other studies involving shower aerosols did not take into consideration the exposure differences between the person in the shower and another within the bathroom area. This study showed a significantly lower concentration of bacteria downstream of the actual shower unit. Generally, lower downstream concentrations were expected due to two factors. First, as the aerosols exit the shower stall and further mix with test chamber air, there is an increased degree of dilution effectively reducing the bioaerosol concentration. Second, the concentration of particles in the shower rises quicker than in the downstream sampling section; this lag time also contributes to the differences observed between the results at the two locations. Furthermore, because of the fragile nature of vegetative cells it is likely that as the bioaerosols mix with ambient air while traveling downstream, the relative humidity decreases, thus the probability of cell desiccation and cell stress are enhanced, thereby reducing cell viability. The difference in viability between in-shower and downstream concentrations was dramatically more pronounced for P. aeruginosa than for B. diminuta under identical conditions, suggesting that P. aeruginosa is considerably more fragile than B. diminuta when aerosolized. There was however no seen differences in concentrations between a forward-facing and rear-facing individual within the shower.
Impact of water temperature.
The present study sought to determine the differences between cold, cool, and hot water temperatures on the concentration of respirable viable bioaerosols generated during showering. Statistical analyses determined that temperature does not significantly affect bioaerosol generation; however a hot shower significantly lowers culturable bioaerosols (10˚C = 25˚C ≠ 40˚C). These findings correlate well with particle mass concentrations from hot and cold showers observed by Cowen and Ollison (2006). Their analysis determined that as the water temperature increased the concentration of particles (based on mass) in the size ranges of 0.5–1.0μm and 1–2.5μm decreased, and the concentration of fine particles (0.3–0.5μm) increased (Cowen and Ollison 2006). Higher temperatures increase the rate of evaporation; therefore, the number of small particles is increased and the culturability of cells is decreased. In another study, Zhou et al. (2007) found that as the water temperature increased the total particle mass concentration within the respirable range (≤10 μm) also increased. O’Toole (2009) observed that with increasing water temperatures aerosol concentrations increased at all particle sizes measured (0.6 to 15μm). The average comfort zone for shower water is generally considered to be between 33–43˚C (Perkins et al. 2009). Therefore, the present study demonstrates that the average shower is taken at temperatures where the number of respirable particulates is increased, but the culturability of aerosolized vegetative cells is decreased. It must be noted that P. aeruginosa has been shown to go into a viable but nonculturable state under stressed conditions (Khan et al. 2010), and thus though its culturability was reduced following aerosolization the overall health implications to showering individuals may not be eliminated.
Importance of B. diminuta and P. aeruginosa for future applications
B. diminuta is often selected as a test organism for aerosol testing due to its small size. However, B. diminuta has also been implicated as the causal agent in occasional infections (Menuet et al. 2008). The clinical significance of P. aeruginosa is much more pronounced; in a 22 month study including over 450 hospitals, P. aeruginosa was the sixth most common identified pathogen (Kerr and Snelling 2009). It has been estimated that as much as 30% of the P. aeruginosa nosocomial pneumonias are contracted through contaminated tap water (Anaissie et al. 2002). P. aeruginosa is known to persist in a wide range of aquatic environments including lakes, rivers, swimming pools, and water distribution systems (Mena and Gerba 2009).
Partitioning coefficients for pathogen can be used to predict the concentration of microorganisms in shower water, biofilms, and air that would result in pulmonary infection. Application of partitioning coefficient of microorganisms (such as Legionella) could be applied in the shower and other aerosol generating environments to predict and evaluate air concentrations in the infection level with the measured parameters in residences, hospitals and indoor/outdoor areas. Development of aerosol-water partitioning coefficients for other microbial species following the framework presented in this paper could be beneficial to predict the impacts of pathogen release for a particular process (e.g., showering) and to estimate potential inhalation doses for waters known to be contaminated with pathogens. The potential release and exposure findings can be beneficial to the medical community, first responders and potentially homeowners to take actions to protect themselves from estimated worst case of exposure (accidental as in the case of Cryptosporidium in Milwaukee or purposefully as in the case of a bioterrorism attack). Future testing and evaluations are needed at a variety of concentrations to assess residential exposure from aerosolization of additional household activities (such as toilet flushing), water quality, and impact of biofilm and mineral formations on distribution system.
SUPPLEMENTAL INFORMATION
Aerosol Homogeneity of Aerosol Generation
Prior to performing the bioaerosol sampler tests, concentration and size distribution of the aerosol was performed to assess the aerosol homogeneity in the testing chamber using PBS. Aerosolized PBS was generated using the Collison nebulizer and the same operating conditions used for the bioaerosol tests. Aerosolized PBS was sampled at each of 16 sampling locations arranged in a planar 4 × 4 rectangular grid in the downstream sampling duct using an Aerodynamic Particle Sizer (APS, TSI, Inc., Shoreview, MN). In addition, a second APS was used to simultaneously sample the aerosol at a reference point in the center of the 4 × 4 grid. The APS samples were collected during a three-minute sampling period and were subsequently analyzed to assess the spatial and temporal variation of the aerosol concentration. Tap water at 25˚C was run though the test shower system to determine the aerosol concentration within the shower and the rate of aerosol generation from the showerhead. An optical particle counter (Climet CI-500, Redland, CA) was used to measure the total concentration of particles emitted from the showerhead at nine locations within three parallel planes perpendicular to the shower curtain. In each of these planes, measurements were taken at a height within the breathing zone of the mannequin, as well as at a height approximately 30 cm above and below the breathing zone. At each height a measurement was taken on the centerline of the shower, near the shower curtain, and near the back wall of the shower parallel to the shower curtain and that the aerosol inhalation exposure is the same regardless of a person’s orientation in the shower.
Aerosol Generation – Preliminary Characterization
A preliminary assessment was conducted to ensure there was a homogenous concentration of shower aerosol within the test shower system. Relative standard deviations of the nine measurements made in each of three planes varied from 6% to 17% suggesting that there were no large variations in the aerosol concentration in the three planes. Likewise, the differences in the average aerosol concentrations measured for the three planes were between 5% and 12% indicating that there were no large variations in the aerosol concentration in front of and behind the mannequin.
Summary of Daily Characterization Test Procedure
Prior to conducting showering testing with microorganism suspensions, the test system was characterized to ensure proper operation and to determine the aerosol formation rates from unspiked tap water under the various testing conditions that was subsequently evaluated with the spore suspensions. For these tests, the liquid flow rate and pressure were set to 6 ± 0.6 L/min and 35 psi, and the test chamber was operated at a total chamber flow rate of 3200 L/min. Prior to starting each shower test, pre-test actions (e.g., tank and aerosol sampler preparations) had been completed, and it was confirmed that the test chamber was operating at its intended flow setting corresponding to 15 air changes per hour, which is the typical for household bathrooms (ASHRAE, 2004). To initiate the showering event, the recirculation valve was turned off as the valve that allowed the source tank to be pumped to the shower head was turned on; this act initiated the shower. At the same time, all aerosol samplers with the exception of the ACI were turned on and their proper function verified visually. Since the ACI samples directly onto growth media, the sampling duration for this sampler needs to be varied as a function of the source water contamination level to avoid overloading the collection plates. Two to three minutes into the shower test, a liquid sample of approximately 25 mL was retrieved directly from the shower line via the bypass valve located outside the test chamber. The shower supply liquid flow rate and pressure were monitored throughout the test to verify that the shower was functioning as desired (6.0 ± 0.6 L/min and 35 psig, respectively, for all tests). Fifteen minutes after being started, the water supply to the shower and the vacuum pumps to all the aerosol samplers were simultaneously shut down. The test runs with each microorganism were conducted in order of increasing concentration to avoid any potential for contamination from higher concentration biocolloids. Between test runs for different microorganisms, the testing chamber, including the water storage and delivery system, were cleaned and decontaminated to prevent cross-contamination.
Footnotes
DISCLAIMER
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency (EPA). Contractor’s role did not include establishing agency policy. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the EPA. The EPA does not endorse any commercial products, services, or enterprises.
REFERENCES
- Allegra S, Leclerc L, Massard PA, Girardot F, Riffard S, and Pourchez J (2016) Characterization of aerosols containing Legionella generated upon nebulization. Scientific Reports. 6:33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaissie EJ, Penzak SR and Dignani MC (2002) The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med 162, 1483–1492. [DOI] [PubMed] [Google Scholar]
- Angenent LT, Kelley ST, St. Amand A, Pace NR, and Hernandez MT (2005) Molecular identification of potential pathogens in water and air of a hospital therapy pool. Proc. Natl. Acad. Sci 102(13): 4860–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TW and Haas CN (2007) Quantitative microbial risk assessment model for Legionnaires’ disease: assessment of human exposures for selected spa outbreaks. J Occup Environ Hyg 4, 634–646. [DOI] [PubMed] [Google Scholar]
- ASHRAE (2004) ASHRAE Standard: Ventilation of Acceptable Indoor Air Quality. Atlanta GAASHRAE Inc. [Google Scholar]
- ASTM. (2001) Standard Test Method for Retention Characteristics of 0.2-μm Membrane Filters Used in Routine Filtration Procedures for the Evaluation of Microbiological Water Quality ASTM D3862–13.
- Barnewall RE, Comer JE, Miller BD, Gutting BW, Wolfe DN, Director-Myska AE, Nichols TL, and Taft SC (2012) Achieving consistent multiple daily low-dose Bacillus anthracis spore inhalation exposures in the rabbit model. Frontiers in Cellular and Infection Microbiology 2, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnewall RE, Benson EM, Brown MA, Fisher DA, Lindsay AS, Simmons AA, and Anderson MS (2015) Characterization of a large animal aerosol exposure system for aerosolizing four strains of Burkholderia pseudomallei. Aerosol Science 84, 21–38. [Google Scholar]
- Bauer H, Fuerhacker M, Zibuschka F, Schmid H and Puxbaum H (2002) Bacteria and fungi in aerosols generated by two different types of wastewater treatment plants. Water Res 36, 3965–3970. [DOI] [PubMed] [Google Scholar]
- Bennett A and Parks S 2006. Microbial aerosol generation during laboratory accidents and subsequent risk assessment. Journal of Applied Microbiology 100:658–663. [DOI] [PubMed] [Google Scholar]
- Bollin GE, Plouffe JF, Para MF and Hackman B (1985) Aerosols containing Legionella pneumophila generated by showerheads and hot-water faucets. Appl Environ Microbiol 50, 1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers RJ, Medley SR, Dickens ML, Hofacre KC, Samsonow MA and van Hoek ML (2013) Transfer and Reaerosolization of Biological Contaminant following Field Technician Servicing of an Aerosol Sampler. J Bioterr Biodef S3: 011. [Google Scholar]
- Chattopadhyay S, and Puls RW (1999) Adsorption of Bacteriophages on Clay Minerals. Environmental Science & Technology 33(20):3609–3614. [Google Scholar]
- Chattopadhyay S and Puls RW (2000) Forces dictating colloidal interactions between viruses and soil. Chemosphere 41:1279–1286. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D, Chattopadhyay S, Lyon WG, and Wilson JT (2002) Effect of Surfactants on the Survival and Sorption of Viruses. Environmental Science & Technology 36(19):4017–4024. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay, S., Hunt, C.D., Rogers, P.J., Swiechichowski, A.L., and Wisneski, C.L. (2004) Evaluation of Biocides for Potential Treatment of Ballast Water. U.S. Department of Homeland Security, U.S. Coast Guard, Report No. CG-D-01–05.
- Chattopadhyay S. (2016) Adhesion and Decontamination of Biological Contaminants in Drinking Water Distribution Systems. Exposure and Health 8(2):199–210. [Google Scholar]
- Clescerl LS, Greenberg AE and Eaton AD eds. (1998) Standard Methods for Examination of Water & Wastewater. Washington DC: Water Environment Federation; American Water Works Association; American Public Health Association. [Google Scholar]
- Collins S, Stevenson D, Bennett A and Walker J (2017) Occurrence of Legionella in UK household showers. International Journal of Hygiene and Environmental Health 220(2):401–406. [DOI] [PubMed] [Google Scholar]
- Cowen KA and Ollison WM (2006) Continuous monitoring of particle emissions during showering. J Air Waste Manag Assoc 56, 1662–1668. [DOI] [PubMed] [Google Scholar]
- Crimi P, Macrina G, Grieco A, Tinteri C, Copello L, Rebora D, Galli A and Rizzetto R (2006) Correlation Between Legionella Contamination in Water and Surrounding Air. Infection Control and Hospital Epidemiology 27(7):771–73. [DOI] [PubMed] [Google Scholar]
- Deloge-Abarkan M, Ha TL, Robine E, Zmirou-Navier D and Mathieu L (2007) Detection of airborne Legionella while showering using liquid impingement and fluorescent in situ hybridization (FISH). J Environ Monit 9, 91–97. [DOI] [PubMed] [Google Scholar]
- Dennis PJ, Wright AE, Rutter DA, Death JE and Jones BP (1984) Legionella pneumophila in aerosols from shower baths. J Hyg (Lond) 93, 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. (2003) Technical Guidance Document on Risk Assessment European Commission Joint Research Centre; Report EUR 20418 EN/2. [Google Scholar]
- Faulkner BR, Lyon WG, Khan FA, and Chattopadhyay S (2003) Modeling leaching of viruses by the Monte Carlo method. Water Research 37:4719–4729. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Lal H and Srivastava A (2015) Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ International 85, 254272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Brinkman MC, Ashley DL, Blount BC, Lyu C, Masters J and Singer PC (2006) Changes in Breath Trihalomethane Levels Resulting from Household Water-Use Activities. Environmental Health Perspectives 114(4):514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishpun SA, Buttner MP, Willeke K, and Mills AL (2007). Sampling for airborne microorganisms In: Husrt CJ, Crawford RL, Garland GRJL, Lipson DA, Stetzenbach LD (Eds.), Manual of Environmental Microbiology, 3rd ed Washington, DC: ASM Press, pp. 939–951. [Google Scholar]
- Hamilton KA and Haas CN (2016) Critical review of mathematical approaches for quantitative microbial risk assessment (QMRA) of Legionella in engineered water systems: research gaps and a new framework. Environ. Sci.: Water Res. Technol 2:599–613. [Google Scholar]
- Keating GA, McKone TE and Gillett JW (1997). Measured and estimated air concentrations of chloroform in showers: effects of water temperature and aerosols. Atmospheric Environment 31(2): 123–130. [Google Scholar]
- Kerr KG and Snelling AM (2009) Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 73, 338–344. [DOI] [PubMed] [Google Scholar]
- Khan NH, Ahsan M, Taylor WD and Kogure K (2010) Culturability and survival of marine, freshwater and clinical Pseudomonas aeruginosa. Microbes Environ 25, 266–274. [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Baron PA, and Willeke K (2011) Aerosol Measurement: Principles, Techniques, and Applications. 3rd ed Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Leclerc H (2003) Relationships between common water bacteria and pathogens in drinkingwater In: The Significance of HPCs for Water Quality and Human Health. Edited by Bartram J, Cotruvo J, Exner M, Fricker C, Glasmacher A World Health Organization, London, UK. [Google Scholar]
- Lee MT, Pruden A and Marr LC (2016) Partitioning of Viruses in Wastewater Systems and Potential for Aerosolization. Environ. Sci. Technol. Lett 3(5):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Mendis N, Trigui H, Oliver JD, and Faucher SP (2014). The importance of the viable but non-culturable state in human bacterial pathogens. Frontiers in Microbiology 5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, Green BJ, Blachere FM, Martin SB, Law BF, Jensen PA and Schafer MP (2017) Sampling and characterization of bioaerosols. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH) Manual of Analytical Methods (NMAM), 5th Edition. [Google Scholar]
- McKonel TE and Howd RA (1992) Estimating Dermal Uptake of Nonionic Organic Chemicals from Water and Soil: I. Unified Fugacity-Based Models for Risk Assessments. Risk Analysis 12(4):543–557. [DOI] [PubMed] [Google Scholar]
- Mena KD and Gerba CP (2009) Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol 201, 71–115. [DOI] [PubMed] [Google Scholar]
- Menuet M, Bittar F, Stremler N, Dubus JC, Sarles J, Raoult D and Rolain JM (2008) First isolation of two colistin-resistant emerging pathogens, Brevundimonas diminuta and Ochrobactrum anthropi, in a woman with cystic fibrosis: a case report. J Med Case Reports 2, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G, Hewitt M, Stevenson D, Walker JT and Bennett AM (2015) Aerosolization of Respirable Droplets from a Domestic Spa Pool and the Use of MS-2 Coliphage and Pseudomonas aeruginosa as Markers for Legionella pneumophila. Appl Environ Microbiol 81, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole J, Keywood M, Sinclair M and Leder K (2009) Risk in the mist? Deriving data to quantify microbial health risks associated with aerosol generation by water-efficient devices during typical domestic water-using activities. Water Sci Technol 60, 2913–2920. [DOI] [PubMed] [Google Scholar]
- Perkins SD, Mayfield J, Fraser V and Angenent LT (2009) Potentially pathogenic bacteria in shower water and air of a stem cell transplant unit. Appl Environ Microbiol 75, 5363–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TM, Gussman RA, Kenny LC and Vanderpool RW (2001) Evaluation of PM2.5 Size Selectors Used in Speciation Samplers. Aerosol Science and Technology 34:422–429. [Google Scholar]
- Rose CS, Martyny JW, Newman LS, Milton DK, King TE Jr., Beebe JL, McCammon JB, Hoffman RE and Kreiss K (1998) “Lifeguard lung”: endemic granulomatous pneumonitis in an indoor swimming pool. Am J Public Health 88, 1795–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CJ and Pitt LM (2006) Infectious disease aerobiology: aerosol challenge methods In Swearengen JR (Ed.), Biodefense: Research Methodology and Animal Models. Boca Raton, FL: CRC Press. [Google Scholar]
- Saini D, Hopkins GW, Chen C, Seay SA Click EM, Lee S, Hartings JM and Frothingham R (2011) Sampling port for real time analysis of bioaerosol in whole body exposure system for animal aerosol model development. J Pharmacol Toxicol Methods 63, 143149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teltsch B, Kedmi S, Bonnet L, Borenzstajn-Rotem Y and Katzenelson E (1980) Isolation and identification of pathogenic microorganisms at wastewater-irrigated fields: ratios in air and wastewater. Appl Environ Microbiol 39, 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ, Webber D, Sellors W, Collinge A, Frost A, Stagg AJ, Bailey SC, Jayasekera, PN, Taylor RR, Eley S, and Titball RW (2008). Characterization and Deposition of Respirable Large- and Small-Particle Bioaerosols. Applied and Environmental Microbiology 74(20):6437–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe A (2007) Assessment of Personal Direct-Reading Dust Monitors for the Measurement of Airborne Inhalable Dust. Ann. Occup. Hyg 51(1):97–112. [DOI] [PubMed] [Google Scholar]
- USEPA (2004) Air Quality Criteria for Particulate Matter (Final Report, Oct 2004). Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- USEPA (2011) Exposure Factors Handbook: 2011 edition Washington, DC: National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency; EPA/600/R-090/052F. [Google Scholar]
- Weschler W and Nazaroff W (2012) SVOC exposure indoors: fresh look at dermal pathways. Indoor Air 22:356–377 [DOI] [PubMed] [Google Scholar]
- Whiley H, Giglio S and Bentham R (2015) Opportunistic Pathogens Mycobacterium avium complex (MAC) and Legionella spp. Colonise Model Shower. Pathogens 4, 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X (2002) Dermal and Inhalation Exposure to Disinfection By-Products in Drinking Water In Graduate Program in Environmental Sciences and Public Health. p.178. New Brunswick, NJ: Rutgers University. [Google Scholar]
- Xu X and Weisel CP (2003) Inhalation exposure to haloacetic acids and haloketones during showering. Environ Sci Technol 37, 569–576. [DOI] [PubMed] [Google Scholar]
- Xu X and Weisel CP (2005) Human respiratory uptake of chloroform and haloketones during showering. J Expo Anal Environ Epidemiol 15, 6–16. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Benson JM, Irvin C, Irshad H and Cheng YS (2007) Particle size distribution and inhalation dose of shower water under selected operating conditions. Inhal Toxicol 19, 333342. [DOI] [PMC free article] [PubMed] [Google Scholar]


