Figure 3.
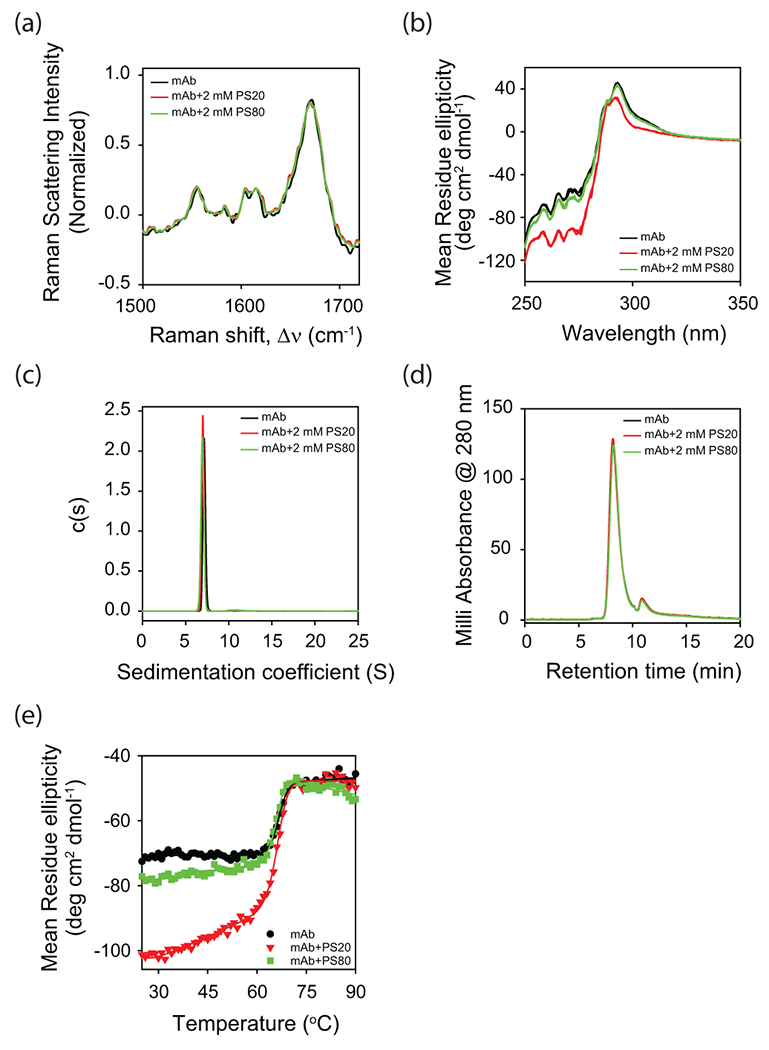
Effect of polysorbate binding on (a) the secondary structure of mAb as probed by Raman scattering, and (b) the tertiary structure of mAb as probed by near-UV CD. Both (c) analytical ultracentrifugation (AUC) and (d) size exclusion chromatography (SEC) indicate that mAb is a monomer in the absence and presence of polysorbates. The peak in the SEC chromatogram (panel d) at the elution time of 11 min correspond to the histidine present in the buffer. (e) Change in the near-UV CD signal as a function of increasing solution temperature. Both PS20 and PS80 affect the partial protein unfolding with relatively no change in the global unfolding of mAb. In all the panels, black, red, and green curves represent the data in the absence of polysorbates, with PS20, and with PS80, respectively. In panel b, triplicate data has been shown for each experimental condition to indicate the statistical significance of the changes in spectra with the addition of polysorbates.
