Figure 2. Memory encoding induces plasticity of inhibitory microcircuit transmission.
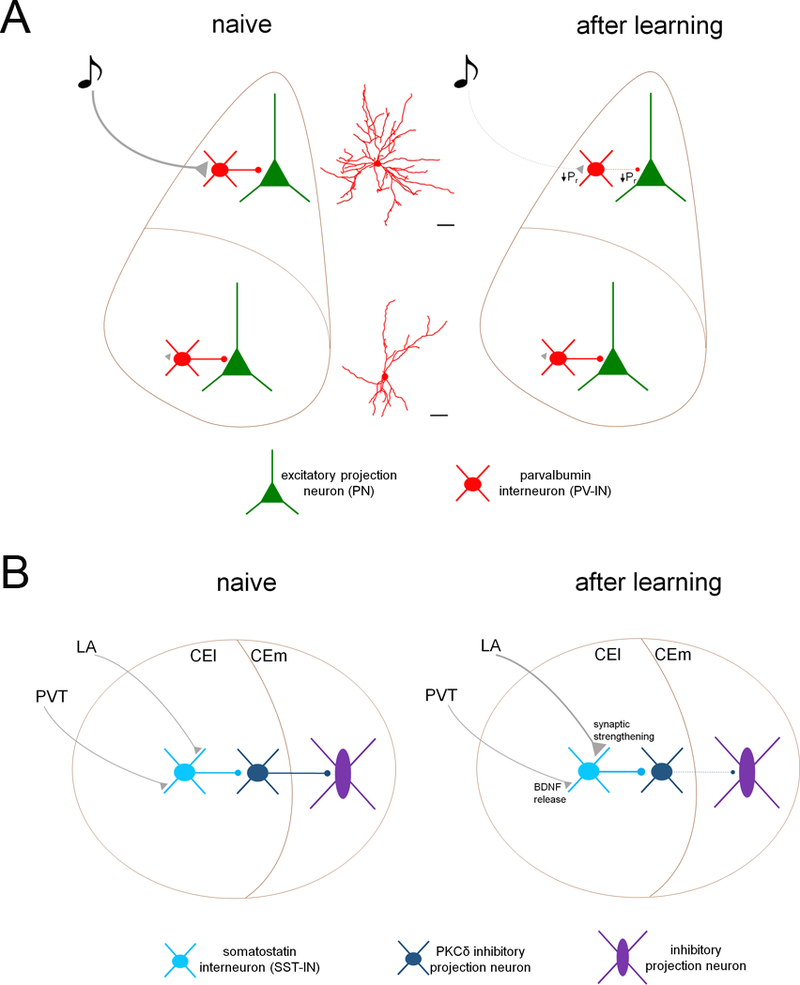
A. PV-INs in the lateral nucleus of the amygdala (LA) receive potent excitation from sensory afferents, likely facilitated by their elaborate dendritic arbors, and drive feedforward inhibition onto excitatory projection neurons (PN). After fear memory encoding, probability of neurotransmitter release (PR) is decreased from sensory afferent terminals onto PV-INs in LA. In turn, PR at PV-IN → PN synapses is also reduced, resulting in persistent LA disinhibition. Conversely, PV-INs in basal nucleus of the amygdala do not drive feedforward inhibition from sensory pathways onto projections neurons and do not undergo glutamatergic plasticity. B. SST-INs of the lateral division of the central amygdala (CEl) receive excitatory input from the lateral amygdala (LA) and the paraventricular nucleus of the thalamus (PVT). After aversive memory encoding, glutamatergic input from LA onto SST-INs is potentiated through enhanced glutamate release from LA axon terminals as well as an increase in postsynaptic AMPA receptor function. These effects are modulated by brain-derived neurotrophic factor (BDNF) release at PVT terminals. This enhanced excitation of SST-INs disinhibits brainstem-projecting inhibitory neurons in the medial nucleus of the central amygdala (CEm) through suppression of firing in PKCδ neurons.
