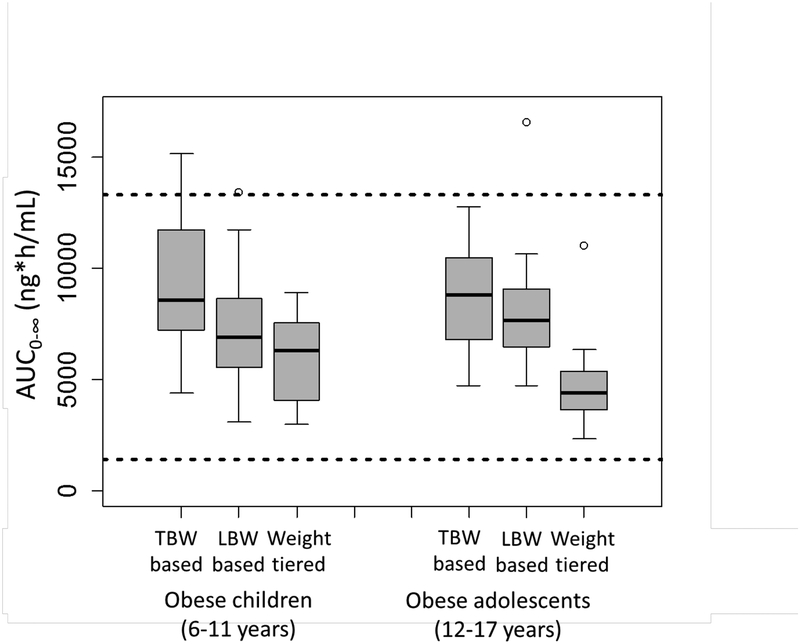
Figure 6.
Pantoprazole AUC0−∞ predicted using population pharmacokinetic model in obese children (n=18) and adolescents (n=19) following total body weight (TBW)-based dosing (1 mg/kg TBW), lean body weight (LBW)-based dosing (1.2 mg/kg LBW), and FDA-approved weight-tiered pantoprazole dosing (20 mg for weight 15–39 kg and 40 mg for weight ≥40 kg), compared to published reported values in adults. Dashed lines represent the range of data from a one-time oral administration of 40 mg pantoprazole to adult extensive metabolizers. The total body weight (median [range]) was 53.9 (32.4–123.4) for obese children, 98.6 (67.2–131.6) for obese adolescents, and 79.2 (32.4–131.6) for all.