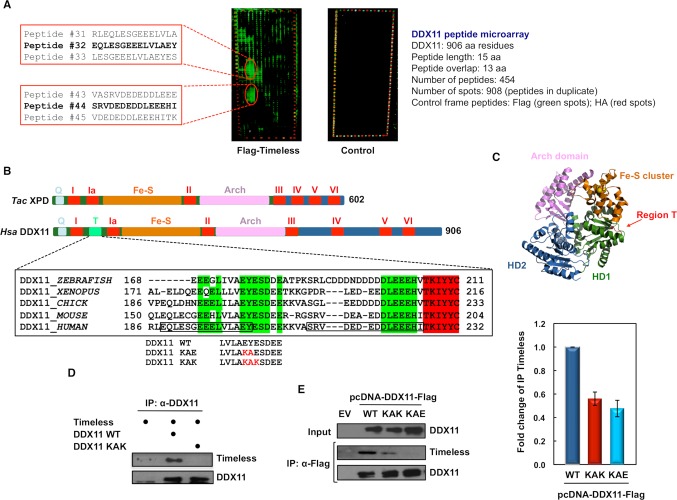
Fig 1. Identification of DDX11 Timeless-binding sites.
A) Overlapping peptide micro-arrays that cover the full-length DDX11 sequence (amino acid residues 1–906) were probed with (on left) or without (control micro-array on right) recombinant purified Flag-tagged Timeless and detected with a mixture of Cy3-labelled anti-Flag antibody and Cy5-labelled anti-HA antibody. Images of the probed arrays obtained with a high-resolution fluorescence scanner are shown. The sequence of DDX11 peptides interacting with Timeless are reported on left. Peptides # 32 and # 44 showing the strongest binding signal in each interaction spot are highlighted in bold. B) Schematic representation of the polypeptide chain of Homo sapiens (Hsa) DDX11 and Thermoplasma acidophilum (Tac) XPD, both belonging to the group of SF2 DNA helicases with a Fe-S cluster. Conserved helicase motifs (from I to VI) are indicated in red. Other sequence motifs are indicated with different colours. Abbreviations used are: Q, for Q motif; Fe-S, for Fe-S cluster; Arch, for Arch domain. A multiple sequence alignment of DDX11 Region T from various vertebrates is reported. Highly conserved residues are in bold; invariant residues are highlighted in green. Adjacent DNA helicase motif Ia is highlighted in red. The sequences of human DDX11 peptides # 32 and # 44 are boxed. Region T mutations to generate the DDX11 KAE and KAK mutants are indicated in red. C) The Tac XPD DNA helicase crystal structure (PDB code: 4a15_A, [29]) is shown. RecA-homology domain 1 and 2 (HD1 and HD2) are in green and blue, respectively. Fe-S cluster and Arch domain are depicted in orange and pink, respectively. Iron and sulphur atoms are shown and coloured in orange and yellow, respectively. Putative position of Region T containing the Timeless-binding sites is shown in red. D) Co-pull down analyses on mixtures of the indicated purified recombinant proteins were carried using an anti-DDX11 antibody bound to Protein A Sepharose beads. Pulled down samples were subjected to immuno-blot analysis to detect the indicated proteins. E) HEK 293T cells were transiently transfected with an empty vector (EV) or a vector expressing Flag-tagged wild type (WT) DDX11 and its mutants (KAK and KAE). 48 hr post-transfection, whole cell extracts were subjected to immuno-precipitation with anti-Flag M2 agarose beads. Western blot analyses of the pulled down samples were carried out and endogenous Timeless was detected using specific antibodies. Immuno-blot experiments were carried out on properly diluted samples and the ImageJ software was used for quantitative analyses of protein bands. Level of immuno-precipitated Timeless was normalized to pulled down Flag-tagged DDX11 in each sample. Means with standard errors of three independent experiments are shown. According to Student’s t-test, a value of P < 0.005 was calculated for the following dataset pairs: Flag-tagged DDX11 WT versus KAK and KAE.