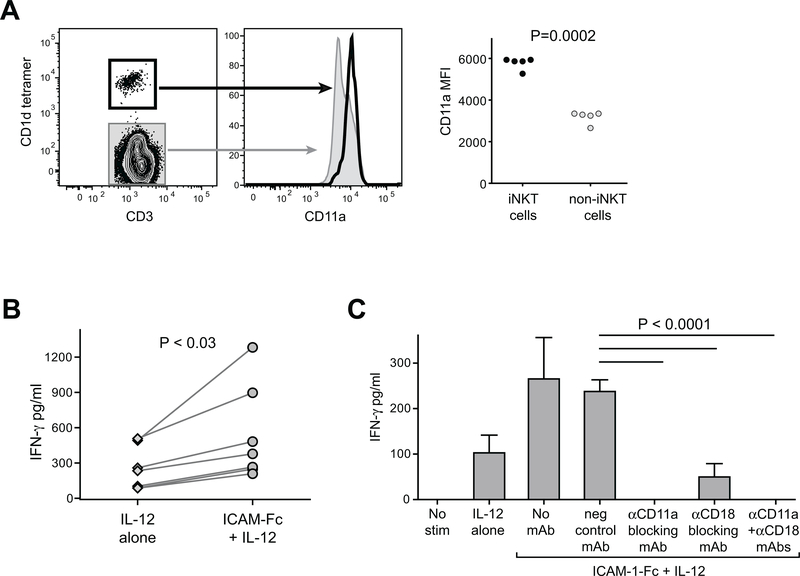
Figure 1. Elevated LFA-1 expression on human iNKT cells co-stimulates IFN-γ secretion in response to IL-12p70.
A) Freshly isolated human PBMCs were stained with antibodies against CD3 and CD11a, and with α-GalCer loaded CD1d tetramer and analyzed by flow cytometry. Plots on left show CD11a expression by CD1d-tetramer positive (heavy black line) compared to tetramer-negative (grey shaded) for one representative experiment. Plot on right shows results from analysis of PBMC samples from five unrelated healthy adults. Mean fluorescence intensity (MFI) of CD11a staining is plotted for CD1d-tetramer positive (iNKT cells) and tetramer-negative (non-iNKT cells). B) iNKT cells were incubated for 24h in medium containing 20 U/ml recombinant human IL-12p70, in the presence of plate-bound ICAM-1-Fc (coated at 5 μg/ml) or negative control mAb (“IL-12 alone”). Secreted IFN-γ was quantitated using a standardized ELISA. The plot shows aggregated results from 7 independent experiments, using 5 different iNKT cell clonal lines (clones PP1.2, PP1.3, PP1.10, J3N.5, GG1.2). C) iNKT cells were incubated for 24h in culture medium alone (“no stim”), or in medium containing IL-12p70 in wells coated with a negative control mAb (“IL-12 alone”), or in medium containing IL-12p70 in wells coated with ICAM-1-Fc in the presence or absence of the indicated blocking antibodies, and secreted IFN-γ was quantitated by ELISA. The plot shows results from one representative experiment out of two using a short-term in vitro expansion of poly-clonal iNKT cells (318D line); bars indicate means and standard deviations from 4 replicates per treatment. Similar results were observed in an additional independent experiments (once with the 318D line, and once with iNKT clone PP1.2).