Figure 4.
The POLE3-POLE4 Complex Binds to H3-H4 Dimers and Tetramers and Promotes Tetrasome Formation and Supercoiling In Vitro
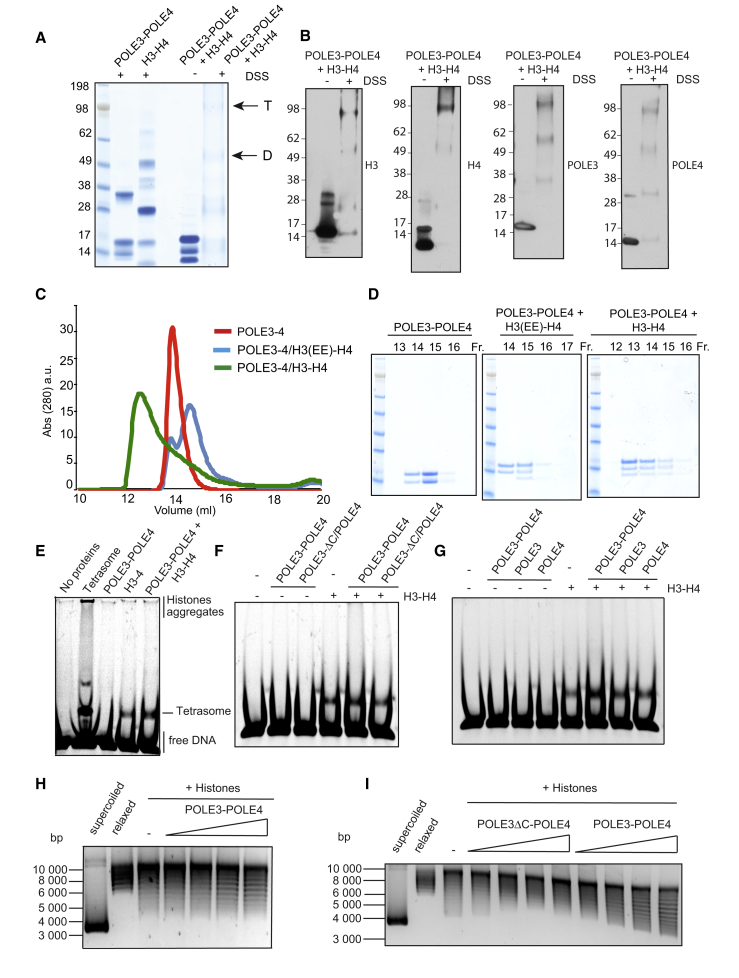
(A) DSS crosslinking experiments performed in 150 mM NaCl. Lane 4 shows control without crosslinking; lanes 1, 2, and 5 show samples incubated with 1 mM DSS for 30 min at 23°C and resolved by SDS-PAGE and Coomassie staining. Lane 3 was left empty. Two black arrows indicate the molecular weight species identified upon crosslinking of POLE3-POLE4/H3-H4 co-complex. T (tetramers) indicates POLE3-POLE4 binding to H3-H4 tetramers, while D (dimers) indicates POLE3-POLE4 binding to dimers.
(B) Western blot analysis of DSS crosslinking experiments performed in 150 mM NaCl. Lane 1 shows control without crosslinking; lane 2 shows sample incubated with 1 mM DSS for 30 min at 23°C. Samples were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and incubated with α-H3, α-H4, α-POLE3, and α-POLE4 antibodies, from left to right.
(C) Analytical gel filtration of POLE3-POLE4 complex alone or in combination with H3-H4 or H3(EE)-H4. Experiments were performed in 300 mM NaCl to prevent histone aggregation.
(D) SDS-PAGE analysis and Coomassie staining of gel filtration fractions from (C).
(E) Tetrasome assembly on linear DNA (Widom 601 sequence) monitored by native PAGE. Lane 2 shows tetrasome preassembled by salt dilution method; lanes 3–5 show linear DNA incubated with the indicated proteins.
(F and G) Tetrasome assembly on linear DNA, performed as in (E), with the indicated proteins.
(H) Plasmid supercoiling assay resolved by native agarose gel electrophoresis. Lane 1 shows supercoiled control phix174 RF1 DNA; lane 2, phix174 RF1 DNA relaxed by TOPO I; and lanes 3–7, phix174 RF1 DNA incubated, in the presence of TOPO I, with histones and increasing concentrations of POLE3-POLE4.
(I) Plasmid supercoiling assay performed as described in (H) using increasing concentrations of POLE3ΔC-POLE4 or POLE3-POLE4.