Abstract
Catharanthus roseus is a commercial source for anti-cancer terpenoid indole alkaloids (TIAs: vincristine and vinblastine). Inherent levels of these TIAs are very low, hence research studies need to focus on enhancing their levels in planta. Since primary metabolism provides precursors for specialized-metabolism, elevating the former can achieve higher amounts of the latter. Cell Wall Invertase (CWIN), a key enzyme in sucrose-metabolism catalyses the breakdown of sucrose into glucose and fructose, which serve as carbon-skeleton for specialized-metabolites. Understanding CWIN regulation could unravel metabolic-engineering approaches towards enhancing the levels of TIAs in planta. Our study is the first to characterize CWIN at gene-expression level in the medicinal plant, C. roseus. The CWINs and their inter-relationship with sucrose and TIA metabolism was studied at gene and metabolite levels. It was found that sucrose-supplementation to C. roseus leaves significantly elevated the monomeric TIAs (vindoline, catharanthine) and their corresponding genes. This was further confirmed in cross-species, wherein Nicotiana benthamiana leaves transiently-overexpressing CrCWIN2 showed significant upregulation of specialized-metabolism genes: NbPAL2, Nb4CL, NbCHS, NbF3H, NbANS, NbHCT and NbG10H. The specialized metabolites- cinnamic acid, coumarin, and fisetin were significantly upregulated. Thus, the present study provides a valuable insight into metabolic-engineering approaches towards augmenting the levels of therapeutic TIAs.
Introduction
Cell Wall Invertase (CWIN, EC: 3.2.1.26), a key enzyme in sucrose-metabolism catalyses the irreversible breakdown of sucrose into glucose and fructose. In addition, it also has several pleiotropic roles such as stress-response, sugar-signalling, flower, fruit and seed development1–3. Besides, CWIN was also found to modulate specialized-metabolites levels in planta4. Introduction of yeast CWIN into Nicotiana tabacum upregulated the levels of phenylpropanoids5. Plant specialized-metabolites had been thought to be of little significance, but advancements in research have unravelled their physiological and therapeutic importance6. Nearly 50,000 therapeutic specialized-metabolites have been identified in plants and characterized to date6,7.
Catharanthus roseus (C. roseus) is a widely-known medicinal plant, used as the predominant source of the pharmaceutically-important specialized-metabolites, especially Terpenoid Indole Alkaloids (TIAs; vincristine and vinblastine are used in anticancer treatment8). Dimerization of vindoline and catharanthine produces vinblastine in planta, which is further converted to vincristine9. The monomeric precursors, vindoline and catharanthine are spatially separated in the plants (vindoline is localized in laticifers and idioblasts whereas catharanthine is secreted to the leaf surface and accumulates in the wax-exudates)10,11. Hence the production of vincristine and vinblastine is limited to trace amounts in planta10. Owing to the inherent low-yields of the anti-cancerous TIAs, various biochemical and molecular studies have been conducted to unravel the specialized-metabolism and enhance TIA concentrations in C. roseus12–14. Industrial production of vincristine and vinblastine is achieved by chemical coupling of more abundant15 monomeric precursors-vindoline and catharanthine16. Therefore, increasing the yields of these precursors in planta, can be a plausible approach to obtain higher yields of the drugs via coupling process.
Most studies have shown that primary and specialized-metabolisms are intimately interconnected, the former providing the precursors to the latter17–19, but to date only a few attempts have been made towards understanding this interconnection, especially at the molecular level20. Sucrose and its hexose products (glucose and fructose) play important roles in both primary and specialized-metabolism. Besides acting as signalling molecules, they also provide carbon skeletons towards the production of specialized-metabolites21. The cross-talk between carbon and specialized-metabolisms has also been reported in glandular trichomes of tomato, wherein the energy and reducing power from photosynthesis are diverted towards specialized-metabolism, achieving high metabolic productivity22. Understanding the interplay between primary and specialized-metabolisms at molecular level involving the important genes and enzymes could unravel novel ways to enable manipulation of specialized-metabolites biosynthesis in planta. Despite the significant role of CWIN in primary and specialized-metabolism, as to our knowledge, so far no work has been done to understand CWIN regulation in any medicinal plants, including C. roseus.
As a part of our study, CWIN genes were identified in C. roseus genome and subjected to in-silico characterization, followed by tissue-specific expression analysis in the leaf, stem and roots. To understand the interrelationship between CWIN and major specialized-metabolism genes in C. roseus, a comparative expression analysis was performed for CWIN and other sucrose-metabolism genes (Sucrose Synthase, SUSY; Sucrose Phosphate Synthase, SPS), TIA pathway genes (Geraniol-10-Hydroxylase, G10H; Deacetylvindoline-4-O-acetyltransferase, DAT; Secologanin synthase, SLS; Peroxidase, PRX1; 1-deoxyxylulose 5-phosphate synthase, DXS; Tryptophan Decarboxylase, TDC; Strictosidine synthase, STR), antioxidants and senescence-associated genes (Catalase, CAT; Superoxide dismutase, SOD and Senescence-associated gene, SAG) under different abiotic stress conditions. The gene-expression results were further supported by metabolite analysis (includes monomeric TIAs: vindoline, catharanthine; bis-indole TIA: vinblastine). Finally, to study the effect of C. roseus CWIN on specialized-metabolism in cross-species, the full-length CWIN isoform (CrCWIN2) was transiently overexpressed in N. benthamiana leaves, followed by gene-expression and metabolite analyses.
Results and Discussion
Identification and in-silico analysis of CWIN isoforms from C. roseus
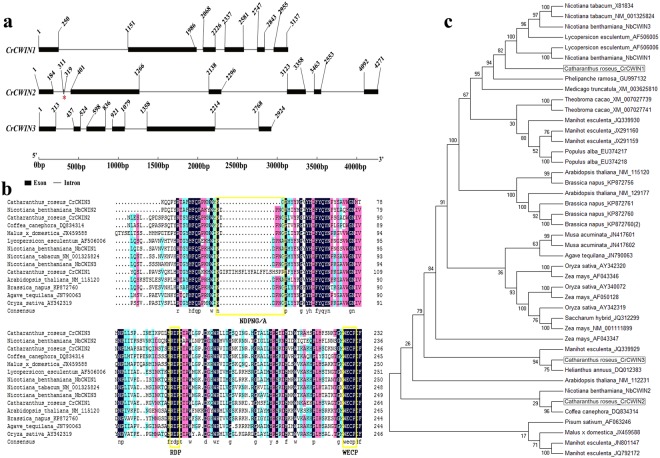
Homology based analysis of the C. roseus coding sequences revealed the presence of three CWIN isoforms; CRO_T000083 (CrCWIN1), CRO_T031716 (CrCWIN2), and CRO_T020329 (CrCWIN3). CrCWIN2 (CDS length: 1725bp; Genomic scaffold: cro_scaffold_3060381) had 7 exons and 6 introns whereas CrCWIN1 (CDS length: 1797bp; Genomic scaffold: cro_scaffold_3070386) and CrCWIN3 (CDS length: 1713bp; Genomic scaffold: cro_scaffold_3065222) were found to have 6 exons and 5 introns each. Previously characterized invertases from Agave tequilana23, Populus trichocarpa24, Sugarcane25 and Cassava26 have been shown to contain 6–8 exons. The genomic architecture of CrCWIN isoforms has been depicted in Fig. 1a.
Figure 1.
(a) Genomic architecture of Cell Wall Invertase isoforms in Catharanthus roseus Solid rectangles represent the exons and the lines represent the introns. The first exon starts with the start codon and the last exon ends with the stop codon. The red asterisk highlights the presence of the second mini exon (9 bp long) in CrCWIN2. Lengths of exons and introns of CrCWIN genes are displayed proportionally as indicated by the scale at the bottom. (b) Multiple alignment of Cell Wall Invertase amino acid sequences from various plants. The important catalytic sites of Cell Wall Invertases, ‘NDPNG’, ‘WECP’ and ‘RDP’ are highlighted in yellow. CrCWIN1 and CrCWIN3 lack the Sucrose-binding box ‘NDPNG’. WECP and RDP are conserved across the isoforms (c). The unrooted phylogenetic tree depecting the evolutionary relationship among CWIN isoforms of C. roseus and other plants. Names and the respective accession id’s. are indicated. Maximum likelihood method was used to construct the tree with 1000 bootstrap replicates using MEGA7 software the C. roseus CWIN isoforms are highlighted with asterisks.
The deduced amino acid sequences of CrCWIN1, CrCWIN2 and CrCWIN3 were predicted to contain 598 (67.8 kDa), 574 (65.0 kDa), 570 (64.9 kDa) amino acid residues. All the isoforms were predicted to localize in the cell wall. These findings have been summarized in Table 1. It is known that CWIN from Sugarcane, SoCIN1 encodes a protein 577 amino acids in length25 and Arabidopsis thaliana CWIN with seven exons and six introns encodes 584 amino acids with mass 66.280kDa27, thus highlighting the molecular similarities among CWINs from C. roseus and other plants.
Table 1.
Cell Wall Invertases in Catharanthus roseus.
| Gene Name (Sequence ID) | Scaffold | Matching sequence details (Genbank ID) | % Identity | Query coverage in tBLASTx | Length of coding sequence (in bp) (Position in scaffold) | Length of genomic gene (in bp) | Predicted amino acid length (Molecular weight; kDa) | Predicted sub-cellular localization |
|---|---|---|---|---|---|---|---|---|
| CrCWIN1 (CRO_T000083) | cro_scaffold_3070386 | Nicotiana tabacum beta-fructofuranosidase, (XM_016633086) | 77% | 95% | 1797 (3502 to 6638) | 3137 | 598 (67.8) | Cell wall |
| CrCWIN2 (CRO_T031716 | cro_scaffold_3060381 | Coffea canephora cell-wall invertase (DQ834314) | 78% | 90% | 1725 (12947 to 17217) | 4271 | 574 (65.0) | Cell wall |
| CrCWIN3 (CRO_T020329) | cro_scaffold_3065222 | Chicorium intybus mRNA for putative invertase (Y11124) | 61% | 90% | 1713 (27037 to 24482) | 2924 | 570 (64.9) | Cell wall |
CrCWIN1 and CrCWIN3 lack the ‘mini-exon’, generally present as the 9 bp long second exon in all the functional CWINs23,28. This exon encodes ‘DPN’, the tripeptide core of the beta-fructofuranosidase motif, ‘NDPNG’ (sucrose-binding box, directly involved in the catalysis of sucrose-cleavage23). Such “defective invertases” lacking the NDPNG motif are thought to be ubiquitous in plant kingdom and are commonly found in tobacco, rice, maize, potato, poplar and chicory. They are known to possess regulatory functions during pollen development29. The other two important catalytic sites, ‘WECP’ and ‘RDP’28 were present in all the three isoforms (Fig. 1b). The Cys-residue of ‘WECP’, is a conserved feature of CWINs28.
The evolutionary relationship among CWINs of C. roseus and other plant species was analysed via phylogenetic analysis (MEGA7). CrCWIN1 grouped with CWINs of L. esculentum and N. tabacum; CrCWIN2 was found to be closely related to C. canephora CWIN whereas CrCWIN3 was found to group with CWIN of H. annuus, with well supported bootstrap values (Fig. 1c).
Tissue specific expression profiling of C. roseus CWIN isoforms
The expression pattern of CrCWINs was analysed in leaf, stem and roots via qRT-PCR followed by LinReg PCR analysis. SAND was used as the internal reference gene30. The result, as shown in Fig. 2 depicts the mean relative expression levels of each isoform in these tissues. Overall, CrCWIN2 (the isoform containing all the catalytic sites) showed the highest expression, followed by CrCWIN3 and CrCWIN1. Highest transcript levels of CrCWIN2 was seen in root tissues (mean relative expression ratio: 11.18), followed by leaves (0.73) and stem (0.24). CrCWIN3 was found to have a similar trend wherein its highest expression was seen in roots (2.54), followed by leaves (0.51) and stem (0.166). High demand for hexoses in roots (sink tissues)28,31 is a plausible reason for the high transcript levels. A similar trend was seen in carrot, wherein the acid invertase activity correlated with the utilization and storage of sugars in sink organs28. In comparison to other two isoforms, the expression of CrCWIN1 was found to be very minimal. Similar tissue-specific differential expression was also observed among maize CWINs wherein Incw3 showed varied expression while Incw4 was constitutively expressed32.
Figure 2.
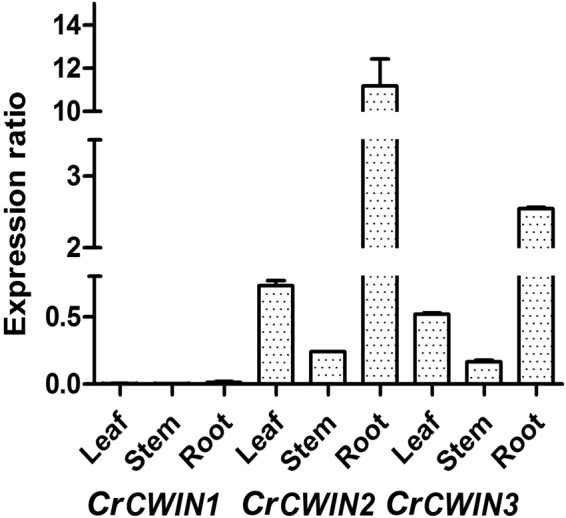
Tissue- specific expression pattern of the three CWIN isoforms in leaf, root and stem tissues of three month old C. roseus plants. The relative expression levels of CWIN isoforms in leaf, root and stem tissues were normalized against transcript levels of SAND. Results are represented as mean relative transcript levels and the error bars indicate standard deviation of triplicate samples.
Stress mediated gene expression profiling in C. roseus
TIA metabolism; specifically vindoline and catharanthine biosynthesis is known to be influenced by abiotic stresses13. Extensive research has been done towards understanding transcriptional responses of TIA biosynthesis genes under conditions influencing alkaloid metabolism33,34. Multiple RNA-Seq experiments have been conducted to understand transcriptomic-modulations under various conditions35–42. Gongora-Castillo et al.36 generated C. roseus transcriptome sequence and expression profiles, wherein it was found that vinblastine biosynthesis genes were up-regulated in response to methyl jasmonate treatment. Sun et al.41 investigated the transcriptional responses to Anthranilate Synthase (AS) overexpression in transgenic C. roseus hairy roots. It was found that aromatic amino acid, fatty acid, glutathione and alpha-linolenic acid metabolism-genes were significantly up-regulated, whereas glycolysis/gluconeogenesis, amino and nucleotide sugar, starch-sucrose, cysteine-methionine and pyruvate-metabolism genes were downregulated, indicating the possible modulations in primary and specialized metabolic pathways due to AS overexpression. Liu et al.39 studied the transcriptomic responses of C. roseus to Peanut-Witches’-broom Phytoplasma-infection via transcriptome sequencing. It was found that many of the abiotic and biotic stimulus- related genes as well as photosynthesis, chloroplast development and energy metabolism genes were up-regulated, indicating at the dynamic changes in primary metabolism and stress related gene-expression. Van Moerkercke et al.35 constructed CathaCyc, a metabolic pathway database of C. roseus, based on RNA-Seq data. Though gene-expression studies have been conducted in C. roseus, the correlation between the expression patterns of CWINs and TIA biosynthesis genes has not been investigated.
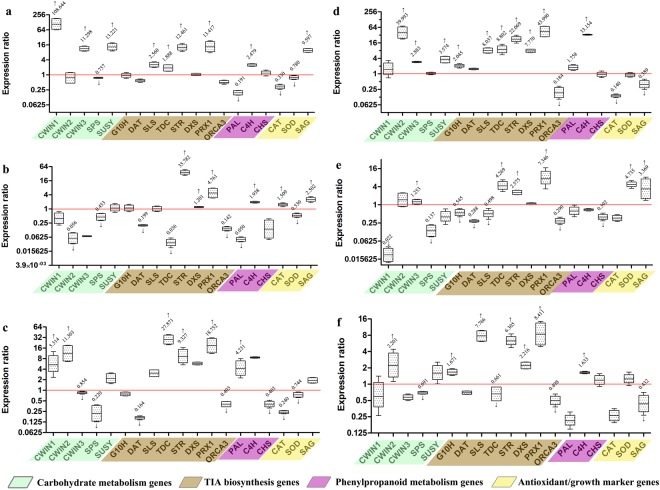
In the present study, C. roseus leaves were subjected to cold, drought, salinity, UV radiation, wounding and also exogenous sucrose treatment. The expression pattern of major genes involved in TIAs biosynthesis was monitored. Also, to study the simultaneous effect on other metabolic pathways, carbohydrate, phenylpropanoid metabolism and antioxidants/growth-associated genes were analysed. All the expression ratios have been depicted in Fig. 3 and the statistically significant (P < 0.05) results are detailed below.
Figure 3.
Gene expression profiles of soluble sugar, TIA metabolism genes, phenylpropanoid metabolism and antioxidant genes. Expression profile in (a) cold stress, (b) drought, (c) salinity, (d) sucrose, (e) UV radiation and (f) wound- treated tissues. The Results depict statistically significant (p < 0.05) Up/downregulation of the considered genes determined via three independent replicates in qRT-PCR. Data were analysed using LinREG PCR and REST software. Mean factors of gene expression compared to control group are represented as boxplots. Corresponding expression ratios of the genes significantly affected (p < 0.05) are shown next to the whisker-boxes. The median expression ratio values above/ below 1.0 indicate up/ downregulation of the target gene under stress treatment compared to the control leaves, indicated using upward and downward arrows.
Cold stress
As shown in Fig. 3a, cold stress resulted in the upregulation of sucrose-metabolizing genes (CrCWIN1, CrCWIN3 and SUSY), whereas SPS was downregulated. Sugars such as glucose, fructose, sucrose, raffinose and stachyose43 are well-known cryoprotectants, mainly involved in protecting cell-membrane integrity by reducing freeze-induced dehydration44,45. Cold-responsive upregulation of SUSY and CWIN isoforms has been previously documented46. While previous studies have shown an upregulation of SPS under cold stress44,46 our results showed a slight decrease in its expression, probably owing to species-specific differences. In response to low temperature stress, plants modulate the expression of genes involved in soluble sugar metabolism and transport, and also starch breakdown45,46, thereby accumulating sugars including sucrose and hexoses that act as cryoprotectants. Thus, a cascade of sugar metabolism genes, transporters and signalling components (such as kinases) is involved in cold stress-response in planta. The differential expression patterns of these genes vary in a species-specific manner47. As for the TIA metabolism genes, SLS, TDC, STR and PRX1 were induced significantly, indicating at a possible role of TIAs in cold stress response. Peroxidases are known to impart cold-tolerance48. A differential expression pattern was observed for the phenylpropanoid-biosynthesis genes. Phenylpropanoids, specifically flavonoids impart freeze tolerance by preventing protein aggregation49. A similar report in A. thaliana presented a slightly differing pattern, wherein PAL was found to be upregulated along with most of the carbohydrate metabolism genes50. Interestingly, the antioxidant gene CAT and SOD were downregulated while SAG was upregulated, indicating that PRX1 may have a more dominant anti-oxidant role compared to CAT and SOD.
Drought stress
Drought stress was found to have adverse effects wherein most of the tested genes were downregulated (Fig. 3b). The carbohydrate metabolism genes were mostly downregulated. Drought inhibits plant growth, disturbs mineral-nutrient relations and impairs metabolism due to changes in photosynthetic carbon metabolism51,52. While two of the TIA-biosynthesis genes (DAT and TDC) were repressed, significantly high upregulation was observed for STR and PRX1, probably attributed to the increased demand of turgor pressure53. The phenylpropanoid biosynthesis genes again showed a differential expression pattern. Elevated levels of phenolics and their biosynthesis genes is a characteristic of drought-stressed tissues53. Cell wall toughening during drought was associated with enhanced lignin (a derivative of phenylpropanoid pathway) biosynthesis53–55. The antioxidant genes showed a differing trend, while SAG was elevated under drought. These observations are in agreement with previous report wherein it was found that drought stress causes modulations in C/N ratio, decreased growth and senescence onset in sorghum56.
Salinity
As shown in Fig. 3c, salinity had varying effects on the expression of sucrose-metabolism genes, wherein CrCWIN1 and CrCWIN2 were highly induced, while CrCWIN3 and SPS were repressed. Osmoregulation is a key aspect in salinity tolerance in plants and some of the major osmolytes like proline, sugars and polyols play pivotal role in alleviating salt stress, thus explaining the elevated levels of sugar metabolism genes57. Among the TIA metabolism genes, TDC, STR and PRX1 were upregulated, while DAT was downregulated. Alkaloids are known to impart salinity tolerance to plants further corroborating their role in alleviating salinity stress58,59. Our results indicating the elevated levels of CWINs, STR, TDC and PRX1 under salinity therefore provide a prospective molecular-crosstalk between carbohydrate and TIA-biosynthesis pathways. The phenylpropanoid gene PAL was induced, while CHS was repressed. Similar reports have indicated that salt treatment could upregulate phenylpropanoid biosynthesis genes in safflower60, Salvia species61 and Caragana korshinskii62.
Sucrose-supplementation
Exogenous sucrose treatment had a marked effect on the soluble sugar, phenylpropanoid and TIA metabolism genes, wherein the expression of most of the genes examined was found to be upregulated (Fig. 3d). Sucrose treatment resulted in a highly pronounced upregulation of CrCWIN2, but not of CrCWIN1 or CrCWIN3, possibly due to the lack of the sucrose-binding box in these two isoforms. Further, SUSY was also found to be upregulated. The TIA-biosynthesis genes, G10H, SLS, TDC, STR, DXS and PRX1 were found to be significantly induced. Among the phenylpropanoid genes, PAL and C4H were considerably upregulated. It has been suggested that sucrose-supplementation induces CWIN in potato, along with principal phenylpropanoid genes, caused by a network of transcription factors (WD40, AN1 and bHLH63). Sucrose-supplementation has been known to improve the therapeutic TIAs and phenylpropanoids64. Further, CAT and SAG were repressed, indicating a possible reduction in oxidative stress and senescence.
UV stress
As shown in Fig. 3e, UV treatment downregulated CrCWIN1, while TIA metabolism genes showed a varying trend. G10H, DAT and SLS were downregulated, while TDC, STR and PRX1 were upregulated. Previous reports also found an upregulation in the expression of STR and TDC65. UV-mediated alkaloid enhancement is attributed to their UV-absorbing properties, which prevents the damage to photosystems caused by UV-B radiation45,66. Further, CHS was considerably downregulated, which is in agreement with the previous observation in A. thaliana wherein several phenylpropanoid metabolism genes were downregulated in plants exposed to UV radiation67. However, the sensitivity of plants to UV radiation has been shown to vary with different plant species68. The antioxidant and senescence marker genes SOD and SAG were upregulated, possibly indicating elevated demand for ROS scavenging mechanisms due to UV stress.
Wounding stress
As depicted in Fig. 3f, wounding stress resulted in a slight upregulation of CrCWIN2, while repressing SPS, indicating a possible enhancement in sucrose breakdown and reduction in its synthesis. Remarkably, sugars are known to regulate the expression of wound-inducible genes, such as pathogenesis-related genes69, thereby corroborating the influence of wounding on soluble sugar metabolism genes. TIA-biosynthesis genes (SLS, STR, DXS and PRX1) were largely upregulated. In C. roseus, wounding is known to activate MAP-K mediated signalling cascade and subsequently, the genes and regulators of TIA-biosynthesis pathway33. Our results further indicated that except C4H, all the phenylpropanoid biosynthesis genes were repressed. This observation showed that resource allocation might be directed towards lignin biosynthesis in response to wounding.
TIA metabolism in C. roseus is under tight regulation at transcriptional level by several transcription factors such as ORCAs, CrBPF1, CrWRKY1, CrMYC1, CrMYC2, BIS1, GBF2 and ZCTs70. CrWRKY1 binds to the promoter of TDC and its overexpression resulted in the upregulation of several genes, especially regulating the serpentine pathway. CrBPF1 (a MYB transcription factor) is known to repress TIA levels. CrGBF1, CrGBF2 and the Zinc Finger Transcription factors- ZCT-1, 2, 3 are known transcriptional repressors of TIA biosynthesis. ORCA3, an AP2/ERF factor is a master regulator of primary and specialized metabolism in C. roseus71 and is known to play critical role in TIA biosynthesis70. ORCA3 transactivates the expression of Strictosidine synthase (STR), a key TIA biosynthesis gene by binding to its 5′ upstream-cis-element, jasmonate and elicitor-responsive element (JERE)71. Further, it upregulates the expression of several structural genes such as TDC, D4H, SLS, CPR, DXS and AS)71. Considering the importance of ORCA3 in TIA biosynthesis, its expression profile was monitored in our study. Remarkably, ORCA3 was found to be downregulated in sucrose, UV, salt and drought treated C. roseus leaves, while in cold and wounding, there was no significant change observed. However, the TIA-biosynthesis genes were found to be upregulated under these conditions, indicating the possibility of additional regulatory components besides ORCA3, operating under these conditions.
To summarize, sucrose treatment was found to simultaneously upregulate CrCWIN2 and major specialized-metabolism genes, along with downregulation of antioxidant systems (CAT) and senescence marker (SAG), indicating a reduction in oxidative stress and senescence. This observation points at a possible pattern of co-regulation in primary and specialized-metabolism gene networks in response to sucrose feeding in C. roseus leaves.
Metabolite analysis of stress-treated leaf tissues
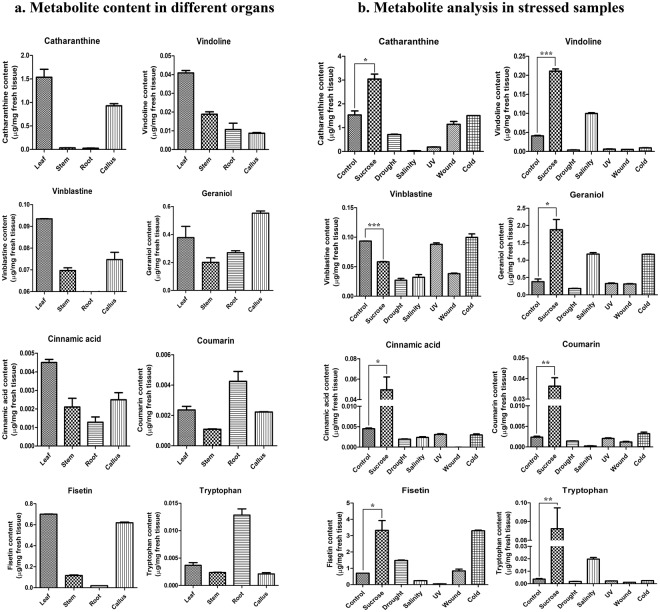
Plant metabolome undergoes profound changes in response to abiotic stress72. In order to study the effect of stress treatments on specialized-metabolites in C. roseus, we assessed the levels of the monomeric precursors of anticancer TIA’s-vindoline and catharanthine, along with the bis-indole alkaloid-vinblastine, cinnamic acid (product of PAL catalysed reaction), coumarin, fisetin (phenylpropanoids) and geraniol (a monoterpenoid-alcohol with limiting role in TIA-biosynthesis73). C. roseus leaves were found to contain highest amounts of the therapeutic TIAs (Fig. 4a), therefore the leaf tissues were selected for analysing changes in metabolite amounts under the selected conditions.
Figure 4.
Quantification of TIAs (vindoline, catharanthine and vinblastine), specialized metabolites (geraniol, cinnamic acid, coumarin and fisetin) and tryptophan (TIA precursor amino acid) in the (a) leaf, stem, root and calli of C. roseus as well as (b) leaves subjected to different conditions influencing TIA metabolism: sucrose supplementation, drought, salinity, UV, wounding and cold stress. The error bars represent standard deviation of duplicate measurements. Statistical significance between sucrose-treated and control samples was tested using student’s t-test (*<0.05; **<0.01; ***<0.001).
It was observed that cold treatment led to a significant decrease in vindoline, while no change was observed in the levels of catharanthine. In a previous report34, catharanthine and vindoline accumulation was shown to be downregulated in response to cold. A precursor of TIAs, geraniol was found to be enhanced in cold-treated leaves. However, a previous report on geranium indicated that low temperature decreased geraniol levels, although the precise mechanisms are unclear74. Phenylpropanoids form the first line of defence against abiotic stress, owing to their inherent antioxidant potential75. Accumulation of cinnamic acid was found to be decreased. These results further correlate with the expression profile of phenylpropanoid genes, wherein PAL was found to be downregulated. However, existing reports present contrasting findings, indicating the species-specific nature of phenylpropanoid regulation under low temperature stress76–78. There was no change observed in the levels of coumarin. In Arabidopsis leaves, the levels of scopoletin, a coumarin-derivative was found to increase in response to cold treatment79. The differences in observations can be due to the differences in metabolic reorganization of individual plants in response to stress. Cold stress resulted in a remarkable increase in the levels of fisetin. Flavonoids are known to accumulate in response to abiotic stresses, thereby conferring tolerance to low temperatures49,80.
Drought stress led to a decrease in levels of all the metabolites analysed, except for fisetin. Drought has been shown to cause dynamic variations in the levels of vindoline and catharanthine, wherein vindoline displayed a decline-rise trend while catharanthine showed a gradual decline in its levels in C. roseus tissues subjected to PEG-induced drought stress81. Geraniol content was found to decrease upon drought treatment. The levels of geraniol in plants was shown to depend on the intensity of drought stress, duration and the species82. Cinnamic acid levels were found to be decreased, which correlate with our gene-expression data, wherein PAL was significantly downregulated. However, previous reports have shown contrasting results83,84. Accumulation of fisetin showed a marked increase in drought-treated tissues, indicating a possible drought-mediated upregulation of flavonoid biosynthesis. A differential effect of drought on flavonoid biosynthesis was reported in wheat85. Flavonoids act as ROS quenchers, thereby forming first-line of defense against oxidative stress86. Coumarin levels were found to be decreased. A similar result was observed in case of Vitis vinifera leaves, wherein most of the abundant phenolic compounds underwent a significant decline, despite other reports indicating at the converse, owing to the species-specificity of drought-induced changes in metabolite levels87.
Salinity stress resulted in a significant increase in the levels of vindoline, while catharanthine was severely reduced. Previous reports suggest conflicting effects of salinity on TIA levels34,88. Geraniol concentration increased in salt-treated tissues, thereby pointing at an upregulation of monoterpenoid biosynthesis. A similar observation was made in Coriandrum sativum, attributed to the increased density of oil glands89. Cinnamic acid concentrations showed a significant decrease in salt-treated tissues. However, a differential effect of salt stress on PAL isoforms was observed in diverse plant species60,84,90,91. It could therefore be inferred that the effect of salinity on cinnamic acid is species-dependent. The levels of fisetin showed a drastic decline under salinity. Research reports have indicated that pattern of flavonoid accumulation under salinity stress is species-specific, governed mainly via the predominant flavonoid present92–94.
UV stress resulted in a downregulation of TIAs and phenylpropanoids. On the contrary, previous studies have reported UV based induction of TIAs under Nitrogen-supplementation to C. roseus leaves95. An increase in vindoline and catharanthine levels was also reported in C. roseus suspension cell cultures subjected to UV radiation65. The levels of geraniol and coumarin were not affected by UV stress. UV stress has been shown to have differential effects on geraniol in different plants, attributed mainly to ROS-mediated signalling96. UV radiation has been proposed to induce the biosynthesis of UV-absorbing and ROS-scavenging phenols97 however, we report that the levels of phenylpropanoids either show a decrease (cinnamic acid and fisetin) or no change (coumarin). This could be attributed to plant-specific differences in response mechanisms to UV-exposure.
Wounding stress downregulated the production of catharanthine, vindoline, cinnamic acid and coumarin, whereas geraniol and fisetin were not significantly affected. Alkaloid formation was shown to be reduced in detached plant parts subjected to wound stress in C. roseus, attributed to the developmental-specific regulation98. Vázquez-Flota et al.99 reported an increase in vindoline and ajmalicine levels of wounded C. roseus seedlings, while catharanthine levels remained unaffected. Cinnamic acid esters are known to have wound-protectant effects and phenylpropanoid-derived metabolites such as acetosyringone play roles in wound stress- response100. The observed decline in cinnamic acid levels can be due to the channelling of this compound towards the synthesis of other downstream wound-protectant metabolites.
Exogenous sucrose-supplementation resulted in highly pronounced upregulation in the biosynthesis of all the metabolites analysed. Sucrose can act as signalling molecule inducing the biosynthesis of various specialized-metabolites such as flavonoids and anthocyanins63,101. Though the levels of vindoline and catharanthine were significantly increased upon sucrose treatment, the dimeric alkaloid, vinblastine was not upregulated upon any of the stress treatments. This could be attributed to the spatial separation of its precursors, vindoline and catharanthine in the leaf tissues10,11. Previous attempts towards enhancing TIAs in C. roseus have also shown upregulation of the monomeric precursors, rather than the dimeric TIAs. Overexpression of ORCA3 and G10H had a more pronounced effect on the accumulation of the precursors (strictosidine, vindoline, catharanthine and ajmalicine) than the dimeric TIAs (anhydrovinblastine and vinblastine)12. Transient overexpression of CrMPK3 also resulted in a higher upregulation of vindoline, catharanthine and serpentine compared to vincristine (dimeric TIA)33. Moreover, the levels of vindoline and catharanthine in planta are inherently higher than those of the dimers, thereby enabling researchers to commercialize their in-vitro coupling to obtain the dimeric TIAs102,103. Thus, strategies for increasing the levels of vindoline and catharanthine via sucrose-metabolism in C. roseus, followed by their isolation and chemical- coupling to obtain the dimeric TIAs would be promising towards enhanced production of anti-cancer TIAs.
In summary, sucrose-supplementation could enhance the production of specialized-metabolites in C. roseus leaves without causing damage to growth-associated processes. Further studies to dissect the mechanistic aspects of this effect could open up novel avenues in metabolic engineering of medicinal plants. Figure 4b summarizes the effect of the stress treatments on metabolite accumulation in C. roseus. The chromatograms (recorded at 210 nm, 250 nm and 269 nm) are available in Supplementary Fig. 1a–c.
Isolation, cloning and transient overexpression of CrCWIN2 CDS in N. benthamiana
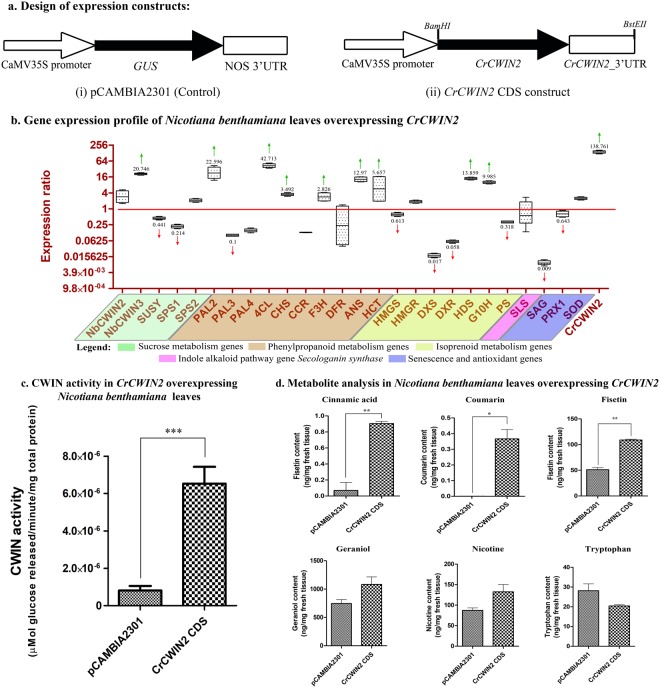
The overexpression of CrCWIN2 in N. benthamiana resulted in 138-fold higher accumulation of CrCWIN2 (Fig. 5a,b). The CWIN activity in infiltrated tissues was found to be ~2.2 times more than the control (N. benthamiana leaves infiltrated with recombinant agrobacterium carrying the vector pCAMBIA2301), thereby validating the functionality of CrCWIN2 (Fig. 5c). The results further showed changes in the expression of endogenous genes belonging to diverse pathways. Most notably, among the sucrose-metabolism genes, NbCWIN3 was significantly enhanced, while NbSPS1 and NbSUSY were downregulated. This pattern suggested that heterologous expression of CrCWIN2 could simultaneously alter the sucrose-synthesis as well as breakdown processes, which are respectively governed mainly via SPS and invertases. Among the phenylpropanoid biosynthesis genes, it was observed that NbPAL2 was upregulated significantly, pointing at the possible role of metabolic-restructuring caused by CWIN overexpression. Further, the genes downstream to PAL revealed an interesting pattern, wherein the lignin-pathway genes (Nb4CL and NbHCT) and anthocyanin-biosynthesis genes (F3H and ANS) were also found to be significantly induced (Fig. 5b). In the isoprenoid-biosynthesis pathway, the mevalonate-biosynthesis genes (NbHMGR, NbHMGS) showed no pronounced changes in their expression, while the non-mevalonate pathway genes (NbDXR, NbDXS) were repressed. However, the downstream genes of isoprenoid biosynthesis pathway: NbHDS, and NbG10H were found to be upregulated (Fig. 5b). This observation further points at the possible interconnection between sucrose-metabolism and other specialized-metabolic processes.
Figure 5.
Heterologous expression of C. roseus CWIN CDS in N. benthamiana. (a) Expression construct carrying the [i] control vector pCAMBIA2301 and [ii] recombinant pCAMBIA2300 with CrCWIN2 CDS downstream to CaMV35S promoter. (b) Expression profile of endogenous genes involved in carbohydrate metabolism, isoprenoid biosynthesis, phenylpropanoid metabolism and growth-associated genes in the N. benthamiana leaves transiently overexpressing CrCWIN2. Upregulation and downregulation have been indicated using an upward (green) and downward (red) arrow respectively. The numbers indicate expression ratios computed via REST© software using pCAMBIA2301 vector-infiltrated sample as control. (c) CWIN activity in control (pCAMBIA2301) versus CrCWIN2 infiltrated N. benthamiana leaves 4-days post infiltration. (d) Quantification of specialized metabolites (cinnamic acid, coumarin, fisetin and geraniol), alkaloid (nicotine) and tryptophan in the N. benthamiana leaves overexpressing CrCWIN2 compared to pCAMBIA2301 infiltrated leaves. Mean and standard deviations (error bars) of triplicate reactions are represented. Statistical significance (P < 0.05) of the differences in means was analysed using t- test.
The metabolite analysis of CrCWIN2 overexpressing N. benthamiana leaves against the agroinfiltrated control showed a significant increase in the levels of specialized metabolites- cinnamic acid, coumarin, and fisetin (Fig. 5d). This indicated that CWIN overexpression could possibly enable partitioning of intermediates towards biosynthesis of specialized-metabolites. A previous study also reported that overexpression of yeast CWIN could enhance the levels of phenylpropanoids5 as an inherent mechanism towards protecting plant systems from pathogen-induced stress. Moreover, research evidence pointed that anthocyanins and flavonoids were recruited mostly under stressed conditions in planta, wherein tissue ROS content is usually higher86.It is also known that the metabolite influx for lignin biosynthesis occurs through sucrose via the shikimic acid and phenylpropanoid biosynthesis pathway104. The chromatograms (recorded at 210 nm, 250 nm and 280 nm) are available in Supplementary Fig. 2.
Conclusion
Primary and specialized-metabolisms in plants are interconnected as primary metabolites can serve as precursors and signals for the synthesis of specialized-metabolites18,19. A clear molecular understanding of this interconnection can lead to novel metabolic engineering approaches for enhancing the biosynthesis of therapeutically important plant specialized-metabolites. C. roseus, the source of anti-cancer TIAs is an important medicinal plant in which the specialized-metabolism, especially TIA-biosynthesis has been extensively studied14. But, the genetic understanding of TIA-biosynthesis in relation to central carbon metabolism is lacking. A sucrose-cleaving enzyme, CWIN plays pivotal roles in modulating diverse specialized-metabolic pathways in planta4. In spite of research reports indicating at the profound effects of CWIN on specialized-metabolites biosynthesis, as to our knowledge, there have been no studies done towards understanding CWIN expression, regulation and its influence on specialized-metabolism in medicinal plants. The present work is the first to understand the possible interrelation between CWIN expression and TIA biosynthesis in the anti-cancer medicinal plant C. roseus.
This study identified three CWIN isoforms in C. roseus, which exhibited tissue-specific differential expression patterns. Among the three isoforms, only one (CrCWIN2) was found to possess the catalytic sites required for invertase functionality. Gene-expression analysis was performed to decipher the possible correlation between the expression patterns of CWIN isoforms, TIAs, phenylpropanoid biosynthesis genes, sucrose metabolism genes and also growth/ antioxidant genes under abiotic stress conditions known to influence vindoline and catharanthine production in C. roseus. Sucrose-supplementation was found to enhance the expression of CWIN and specialized-metabolism genes, and also improved the levels of vindoline, catharanthine, geraniol, fisetin, coumarin and cinnamic acid. The interconnection between primary and specialized-metabolism was further confirmed via transient overexpression of full-length CrCWIN2 in N. benthamiana.
These results can give us cues for further metabolic engineering approaches to enhance the production of medicinally/economically important phytochemicals without compromising the overall plant health and vegetative growth. In this regard, future studies to identify the regulatory factors that can co-regulate CWIN and specialized-metabolism genes can be of interest.
Materials and Methods
Plant materials and stress treatments
Seeds of C. roseus (var. Pacifica Cherry red) were germinated on coco peat and maintained at 25 °C and 65% relative humidity in green house. Two months old C. roseus plants were subjected to different abiotic stress treatments. UV treatment was done by exposing the plants to UV radiation (48 μWcm−2) in LAF for two minutes33. Wounding was performed by damaging ~50% of the leaf lamina with a surgical blade105. Cold stress was induced by incubating the plants at 4 °C105. The detached leaves were subjected to salt stress by dipping them in 200 mM NaCl solution105. Drought treatment was performed by placing the detached leaves on dry blotting paper in petri dishes105. Exogenous sucrose treatment was performed by placing the detached leaves in 90 mM sucrose solution101. The tissues were harvested after 24 hours of stress treatments, snap-frozen in liquid nitrogen and stored at −80 °C.
RNA isolation and Quantitative RT-PCR
To study the tissue-specific expression patterns of CWIN, total RNA was isolated from leaf, stem, root and callus tissues of C. roseus. To examine the stress mediated expression of CWIN along with other genes, RNA was extracted from pooled tissues of C. roseus leaves subjected to stress treatments using Plant RNA isolation kit following the manufacturer’s instructions (MN, Germany). 6 µg of total RNA was subjected to DNase treatment using RNase free DNase (Thermo Scientific, Lithuania) followed by first strand cDNA synthesis using PrimeScript RT reagent kit (TaKaRa, Japan). qRT-PCR was performed on Mastercycler Realplex qRT-PCR instrument (Eppendorf). Reaction mix contained 1 µl of diluted (3.5 times) cDNA, 5pmol each of forward and reverse primer, 1X SYBR green (Roche, Germany) in 7.5 µl reaction. Cycling parameters were: Initial denaturation at 95 °C for 5 min, 40 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 40 s, extension at 72 °C for 30 s followed by final extension at 72 °C for 5 min.
The leaf tissues subjected to stress treatments were used for analysis of the expression levels of primary and specialized-metabolism genes using specific primers listed in Supplementary Table S1. SAND served as the reference gene30. The expression patterns of sucrose-metabolism genes: CWIN, SUSY and SPS along with predominant TIA metabolism genes: G10H, DAT, SLS, PRX1, DXS; Phenylpropanoid metabolism genes: PAL, C4H, CHS, TDC, STR, CAT, SOD, SAG and APETALA2-domain transcription factor ORCA3, (a known transcriptional regulator of TIA-biosynthesis genes106) were monitored.
Reaction efficiencies and Cq values of triplicate qRT-PCR assays were obtained through LinReg PCR software107. Using these values, the relative gene-expression ratios were computed via the Relative Expression Software Tool (REST©). REST© performs randomization tests to determine the expression ratio of a sample, using the efficiency- corrected comparative Cq values. The up/downregulation of a gene is determined by taking into account the individual amplification efficiencies of target and reference genes108.
High-Performance Liquid Chromatography (HPLC) analysis
The HPLC analysis was done as described in Singh et al.102 and Lin et al.109, with modifications. The C. roseus and N. benthamiana tissues were harvested and ground frozen in liquid nitrogen. The samples were sequentially extracted with 1:10 w/v ratio of tissue: solvent in a sequence of chloroform, followed by ethyl acetate and finally methanol each for 30 min with vigorous shaking. The supernatants were collected by centrifugation and freeze dried. The extracts were made upto 1 ml using acetonitrile and pooled together in equal ratios. Catharanthine, vindoline, vinblastine, cinnamic acid, coumarin, fisetin, geraniol and nicotine were used as standards, purchased from Chemfaces, China. The samples were filtered through a 0.22 µm syringe-driven filter and analysed via a reverse-phase HPLC system (Agilent 1260-Infinity, C-18 column 4.7 × 250 mm; 5 µm particle size) at 25 °C. The mobile phase consisted of 0.1% formic acid (A) and acetonitrile (B). The elution profile was as follows: 0 min: 100% A; 0–5 min: 100–70% A; 5–25 min: 70–50% A; 25–28 min: 50–30% A; 28–30 min: 30–100% A; 30–35 min: 100% A. The flow rate was maintained at 1 ml.min−1. The injection volume was 20 µl and the eluent was monitored using a PDA-DAD detector between 190 nm and 400 nm. The concentrations of the selected compounds were calculated by comparing the peak area, retention time (RT) and wavelength of the designated compound and expressed in µg of compound per mg of fresh tissue (C. roseus) and ng of compound per mg of fresh tissue (N. benthamiana).
Identification and bioinformatic analysis of C. roseus CWIN coding sequences (CDS)
The amino acid sequences of the well characterized CWINs of Arabidopsis thaliana (AT3G13790), Nicotiana tabacum (X81834), Coffea canephora (DQ834314), Lycopersicon esculentum (AF506006) and Oryza sativa (AY342319) were used as query sequences to find the CWIN coding sequence isoforms in C. roseus via tBLASTn analysis of Medicinal Plant Genomics Resources (MPGR) Consortium (http://medicinalplantgenomics.msu.edu/). Subsequently, amino acid sequences were deduced using Translate tool of ExPASY. (http://web.expasy.org/translate/) and their molecular features were analysed using EBI-Tools (http://www.ebi.ac.uk/Tools/emboss/).
The homology with known sequences was analysed using BLASTn and BLASTx tools of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Subcellular localization was predicted using Plant-mPloc prediction tool (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/)110. Evolutionary relationships of the sequences were compared using Maximum Likelihood method with thousand bootstrap values employing MEGA7 program. Genomic architecture of introns and exons was obtained using Gene Structure Display Server 2.0 (http://gsds.cbi.pku.edu.cn/)111.
Isolation and cloning of full-length C. roseus CWIN CDS
Full-length C. roseus CWIN CDS was isolated via RT-PCR. Total RNA was isolated from leaf tissues of three month old C. roseus plants using RNeasy Plant Mini kit (Qiagen, Germany) following the manufacturer’s instructions. Subsequently, 3 µg of total RNA was subjected to DNase treatment and cDNA was synthesized using Transcriptor first strand cDNA synthesis kit (Roche, Germany). Full-length C. roseus CWIN CDS was amplified using gene specific primers (LP: 5′-GGATCCATGGCCAATTCTTACATTTGGTTCTTCT-3′; RP: 5′-GGTGACCCTCAATCTCACCATGATGAGAAATAAATTT-3′). Underlined bases contained BamHI and SstI sites respectively. The PCR-amplified CDS was cloned into pGEMT-Easy vector and validated by sequencing, restriction analysis and PCR. Next, the C. roseus CWIN CDS was cloned into the modified expression vector pCAMBIA2300 as BamHI-SstI insert, downstream to CaMV 35S Promoter.
Transient overexpression of C. roseus CWIN CDS in Nicotiana benthamiana
Recombinant pCAMBIA2300 was transformed into Agrobacterium tumefaciens strain EHA105 via freeze thaw method112. Transformed clones were verified by PCR followed by agroinfiltration into N. benthamiana as previously reported113. Leaves infiltrated with recombinant agrobacterium carrying the vector pCAMBIA2301 were used as control. After four days, leaves were harvested, snap frozen in liquid nitrogen and stored at -80 °C until further analysis.
To study the effect of CWIN overexpression on other genes in N. benthamiana, total RNA was extracted from agroinfiltrated tissues. 6 µg of total RNA was used to synthesize cDNA and the gene-expression analysis was carried out via qRT-PCR. PP2A114 was used as an internal reference gene. The N. benthamiana genes analysed in this study were 4-coumarate:coenzyme a ligase (4-CL; Nbv6.1trP58793), Anthocyanidin Synthase (ANS; Nbv6.1trP1132), Cinnamoyl-CoA Reductase (CCR; Nbv6.1trP67697), Chalcone Synthase (CHS; Nbv6.1trP67289), Dihydroflavonol 4-Reductase (DFR; Nbv6.1trP53078), Flavanone 3-Hydroxylase (F3H; Nbv6.1trP67389), Shikimate o-Hydroxycinnamoyltransferase (HCT; Nbv6.1trP21540), Peroxidase 9 (PRX; Nbv6.1trP50659), Catalase Isozyme 1 (CAT; Nbv6.1trP54093), Superoxide Dismutase (SOD; Nbv6.1trP67255), Sucrose Synthase (SUSY; Nbv6.1trP69162), Sucrose Phosphate Synthase isoforms (SPS1; Nbv6.1trP64694, SPS2, Nbv6.1trP56089), Cell Wall Invertase isoforms (CWIN2; Nbv5.1tr6202472, CWIN3; Nbv5.1tr6228617), Phenylalanine Ammonia Lyase isoforms (PAL2, Nbv6.1trP20094; PAL3, Nbv6.1trP49210 and PAL4, Nbv6.1trP56366), Flavanone 3-hydroxylase (F3H; Nbv6.1trP67389), 3-hydroxy-3-methylglutaryl-coenzyme-a-reductase 1 (HMGR; Nbv6.1trP54761), Hydroxymethylglutaryl- synthase-like (HMGS; Nbv6.1trP33093), Probable 1-deoxy-d-xylulose-5-phosphate chloroplastic (DXS; Nbv6.1trP16938), 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR, Nbv6.1trP48271), Geraniol 8-hydroxylase-like (G8H; Nbv6.1trP70636), Cytochrome p450 cyp72a219-like (SLS, Nbv6.1trP5153), 4-hydroxy-3-methylbut-2-en-1-yl diphosphate chloroplastic (HDS; Nbv6.1trP30454), Phytoene Synthase (PS; Nbv6.1trP21364). The gene IDs have been obtained from Benth Genome (http://benthgenome.qut.edu.au). Primers used have been enlisted in Supplementary Table S1.
CWIN activity assay
CWIN activity assay was performed as described previously115 with minor modifications. Briefly, the rapidly harvested leaf tissue was weighed, ground in liquid nitrogen followed by homogenization with 1 ml extraction buffer [all as mol m−3: Hepes-KOH (pH 8·0), 50; MgCl2, 5 Ethylenediaminotetraacetic acid (EDTA), 2; MnCl2, 1; CaCl2, 1; Benzamidine, 1; Dithiotreitol, 1; Phenyl-methylsulphonyl sulphonyl fluoride, 0·1] on ice. The homogenate was centrifuged at 13000 × g for 15 min at 4 °C and the pellet was resuspended in 500 µl extraction buffer. Total protein concentration in the extracts was determined using Bradford method116. The reaction mixture containing 10 µg of total protein, 200 mM sucrose and 50 mM sodium acetate buffer at pH 4.7 was incubated at 37 °C for 30 min. After incubation, reaction was alkalinized by adding 100 µl 1 M Tris-HCL, pH 8 and heated at 85 °C for 3 min. Two blanks were set up to measure acid hydrolysis of sucrose (contained no extract) and endogenous glucose levels (contained no sucrose). The amount of hexoses released was measured enzymatically using Sucrose, D-Fructose, D-Glucose assay kit (Megazyme, Ireland). Activity was expressed as micromoles of hexoses released per minute per milligram of total protein.
Statistics
qRT-PCR data were analysed using REST© and represented as box-and-whiskers plot, with central line indicating median of expression ratio with respect to control; box borders represent 95% confidence intervals and whiskers depict standard error margins. All other data are expressed as mean values and standard deviation of three independent experiments. Statistical significance was evaluated using t-test via GraphPad Prism 5 (GraphPad Software, La Jolla California USA, www.graphpad.com). All the graphs were plotted using GraphPad Prism 5 (GraphPad Software, La Jolla California USA, www.graphpad.com).
Electronic supplementary material
Acknowledgements
The authors are thankful to Department of Science and Technology, Govt. of India (Grant sanction number: SB/YS/LS-188/2014) and Department of Biotechnology, Govt. of India (Grant sanction number: BT/Bio-CARe/02/10078/2013–14) for funding the research. We thankfully acknowledge the HPLC facility provided by Dr. Jayapradha Ramakrishnan, SASTRA Deemed to be University (EMR scheme, SERB: SR/S0/HS/0073/2012). The authors thank SASTRA Deemed to be University for providing the necessary infrastructural facilities.
Author Contributions
The research work was conceptualized and designed by B.S. (corresponding author). N.M.J. and S.A.S. performed the experiments; N.M.J., S.A.S. and S.S. interpreted the data. S.S.R. performed the HPLC. analysis, S.S.R. and S.A.S. performed the chromatography data interpretation. N.M.J., S.A.S., S.S. and B.S. wrote the article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. J. Nishanth and S. A. Sheshadri contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33415-w.
References
- 1.Li Z, et al. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of young fruit. J Exp Bot. 2012;63(3):1155–66. doi: 10.1093/jxb/err329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YH, Offler CE, Ruan YL. Cell Wall Invertase promotes fruit set under heat stress by suppressing ROS-independent cell death. Plant Physiol. 2016;1:163–80. doi: 10.1104/pp.16.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ru L, et al. Transcriptomic and metabolomics responses to elevated Cell Wall Invertase activity during tomato fruit set. J Exp Bot. 2017;68(15):4263–4279. doi: 10.1093/jxb/erx219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proels RK, Hückelhoven R. Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol Plant Pathol. 2014;15(8):858–64. doi: 10.1111/mpp.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumert A, et al. Patterns of phenylpropanoids in non-inoculated and potato virus Y-inoculated leaves of transgenic tobacco plants expressing yeast-derived invertase. Phytochemistry. 2001;56(6):535–41. doi: 10.1016/S0031-9422(00)00422-2. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Garcia S, et al. Current Approaches for Enhanced Expression of Secondary Metabolites as Bioactive Compounds in Plants for Agronomic and Human Health Purposes – a Review. Pol. J. Food Nutr. Sci. 2013;63(2):67–78. doi: 10.2478/v10222-012-0072-6. [DOI] [Google Scholar]
- 7.Ibrahim MH, et al. Primary, Secondary Metabolites, Photosynthetic Capacity and Antioxidant Activity of the Malaysian Herb Kacip Fatimah (Labisia Pumila Benth) Exposed to Potassium Fertilization under Greenhouse Conditions. Int. J. Mol. Sci. 2012;13(11):15321–15342. doi: 10.3390/ijms131115321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moudi M, Go R, Yien CYS, Nazre M. Vinca Alkaloids. Int. J. Prev. Med. 2017;4(11):1231–1235. [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad B. et al. Structural and functional characterization of the Vindoline biosynthesis pathway enzymes of Catharanthus roseus. J. Mol. Model. 24(3), 10.1007/s00894-018-3590-2. [DOI] [PubMed]
- 10.Roepke J, et al. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. PNAS. 2009;107(34):15287–15292. doi: 10.1073/pnas.0911451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bernonville DT, et al. Phytochemical genomics of the Madagascar periwinkle: Unravelling the last twists of the alkaloid engine. Phytochemistry. 2014;113:9–23. doi: 10.1016/j.phytochem.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Pan Q, et al. Overexpression of ORCA3 and G10H in Catharanthus roseus Plants Regulated Alkaloid Biosynthesis and Metabolism Revealed by NMR-Metabolomics. PLoS ONE. 2012;7(8):e43038. doi: 10.1371/journal.pone.0043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujib A, et al. Catharanthus roseus alkaloids: application of biotechnology for improving yield. Plant Growth Regul. 2012;68(2):111–127. doi: 10.1007/s10725-015-0109-z. [DOI] [Google Scholar]
- 14.Alam M. Masidur, Naeem M., Khan M. Masroor A., Uddin Moin. Catharanthus roseus. Cham: Springer International Publishing; 2017. Vincristine and Vinblastine Anticancer Catharanthus Alkaloids: Pharmacological Applications and Strategies for Yield Improvement; pp. 277–307. [Google Scholar]
- 15.Ishikawa H, et al. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J. Am. Chem. Soc. 2009;31(13):4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung, et al. Screening 64 cultivars Catharanthus roseus for the production of vindoline, catharanthine, and serpentine. Biotechnol. Prog. 2011;27(4):937–943. doi: 10.1002/btpr.557. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Luthra R, Sangwan RS. Mobilization of starch and essential oil biogenesis during leaf ontogeny of lemongrass (Cymbopogon flexuosus Stapf.) Plant and Cell Physiol. 1991;32(6):803–811. doi: 10.1093/oxfordjournals.pcp.a078147. [DOI] [Google Scholar]
- 18.Dubey-Shankar V, Bhalla R, Luthra R. Sucrose mobilization in relation to essential oil biogenesis during Palmarosa (Cymbopogon martinii Roxb. Wats. var. motia) inflorescence development. J Biosci. 2003;28(4):479–87. doi: 10.1007/BF02705122. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, et al. System level analysis of cacao seed ripening reveals a sequential interplay of primary and secondary metabolism leading to polyphenol accumulation and preparation of stress resistance. Plant J. 2016;87(3):318–32. doi: 10.1111/tpj.13201. [DOI] [PubMed] [Google Scholar]
- 20.Becerra-Moreno A, et al. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front Plant Sci. 2015;6:837. doi: 10.3389/fpls.2015.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci. 2015;16(11):26378–26394. doi: 10.3390/ijms161125967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balcke GU, et al. Multi-omics of Tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell. 2017;29(5):960–983. doi: 10.1105/tpc.17.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortés-Romero C, Martínez-Hernández A, Mellado-Mojica E, López MG, Simpson J. Molecular and Functional Characterization of Novel Fructosyltransferases and Invertases from Agave tequilana. PLoS ONE. 2012;7(4):e35878. doi: 10.1371/journal.pone.0035878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Gao K, Su X, Rao P, An X. Genome-wide identification of the invertase gene family in Populus. PLoS ONE. 2015;10(9):e0138540. doi: 10.1371/journal.pone.0138540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu JQ, Wang AQ, Huang JL, Yang L-T, Li Y-R. Isolation, characterization and promoter analysis of Cell Wall Invertase gene SoCIN1 from Sugarcane (Saccharum spp.) Sugar Tech. 2015;17(1):5–76. doi: 10.1007/s12355-014-0348-8. [DOI] [Google Scholar]
- 26.Yao Y, et al. Genome-wide identification, 3D modeling, expression and enzymatic activity analysis of Cell Wall Invertase gene family from cassava (Manihot esculenta Crantz) Int J Mol Sci. 2014;15(5):7313–7331. doi: 10.3390/ijms15057313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwebel-Dugué N, et al. Arabidopsis gene and cDNA encoding cell-wall invertase. Plant Physiol. 1994;104(2):809–810. doi: 10.1104/pp.104.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturm A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999;121(1):1–8. doi: 10.1104/pp.121.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy LK, et al. Understanding the role of defective invertases in plants: Tobacco Nin88 fails to degrade sucrose. Plant Physiol. 2013;161(4):1670–81. doi: 10.1104/pp.112.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollier J, Bossche R, Rischer H, Goossens A. Selection and validation of reference genes for transcript normalization in gene expression studies in Catharanthus roseus. Plant Physiol. Biochem. 2014;83:20–25. doi: 10.1016/j.plaphy.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Hirose T, Takano M, Terao T. Cell Wall Invertase in developing rice caryopsis: Molecular Cloning of OsCIN1 and analysis of its expression in relation to its role in grain filling. Plant Cell Physiol. 2002;43(4):452–459. doi: 10.1093/pcp/pcf055. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, et al. Characterization of two members of the maize gene family, Incw3 and Incw4, encoding Cell-Wall Invertases. Gene. 2000;245:89–102. doi: 10.1016/S0378-1119(00)00034-2. [DOI] [PubMed] [Google Scholar]
- 33.Raina SK, et al. CrMPK3, a mitogen activated protein kinase from Catharanthus roseus and its possible role in stress induced biosynthesis of monoterpenoid indole alkaloids. BMC Plant Biol. 2012;12:134. doi: 10.1186/1471-2229-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta A, Sen J, Deswal R. New evidences about Strictosidine Synthase (Str) regulation by salinity, cold stress and nitric oxide in Catharanthus roseus. J. Plant Biochem. Biotechnol. 2013;22(1):124–131. doi: 10.1007/s13562-012-0118-1. [DOI] [Google Scholar]
- 35.Van Moerkercke A, et al. CathaCyc, a metabolic pathway database built from Catharanthus roseus RNA-Seq data. Plant cell physiol. 2013;54.5:673–685. doi: 10.1093/pcp/pct039. [DOI] [PubMed] [Google Scholar]
- 36.Góngora-Castillo E, et al. Development of transcriptomic resources for interrogating the biosynthesis of monoterpene indole alkaloids in medicinal plant species. PloS one. 2012;7.12:e52506. doi: 10.1371/journal.pone.0052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krithika R, et al. Characterization of 10-hydroxygeraniol dehydrogenase from Catharanthus roseus reveals cascaded enzymatic activity in iridoid biosynthesis. Sci Rep. 2015;5:8258. doi: 10.1038/srep08258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Shah N, Garg V, Bhatia S. Large scale in-silico identification and characterization of simple sequence repeats (SSRs) from de novo assembled transcriptome of Catharanthus roseus (L.) G. Don. Plant cell rep. 2014;33.6:905–918. doi: 10.1007/s00299-014-1569-8. [DOI] [PubMed] [Google Scholar]
- 39.Liu L-YD, et al. High-throughput transcriptome analysis of the leafy flower transition of Catharanthus roseus induced by peanut witches’-broom phytoplasma infection. Plant Cell Physiol. 2014;55.5:942–957. doi: 10.1093/pcp/pcu029. [DOI] [PubMed] [Google Scholar]
- 40.Shukla AK, Shasany AK, Gupta MM, Khanuja SP. Transcriptome analysis in Catharanthus roseus leaves and roots for comparative terpenoid indole alkaloid profiles. J Exp Bot. 2006;57.14:3921–3932. doi: 10.1093/jxb/erl146. [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Manmathan H, Sun C, Peebles CA. Examining the transcriptional response of overexpressing anthranilate synthase in the hairy roots of an important medicinal plant. Catharanthus roseus by RNA-seq. BMC plant boil. 2016;16(1):108. doi: 10.1186/s12870-016-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma M, Ghangal R, Sharma R, Sinha AK, Jain M. Transcriptome analysis of Catharanthus roseus for gene discovery and expression profiling. PloS one. 2014;9(7):e103583. doi: 10.1371/journal.pone.0103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuanyuan, M. Yali, Z., Jiang, L., & Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. AJ B. 8(10) (2009).
- 44.Kaplan F, et al. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007;50(6):967–81. doi: 10.1111/j.1365-313X.2007.03100.x. [DOI] [PubMed] [Google Scholar]
- 45.Redondo-Gómez Susana. Molecular Stress Physiology of Plants. India: Springer India; 2013. Abiotic and Biotic Stress Tolerance in Plants; pp. 1–20. [Google Scholar]
- 46.Yue C, et al. Effects of cold acclimation on sugar metabolism and sugar-related gene expression in tea plant during the winter season. Plant mol boil. 2015;88.6:591–608. doi: 10.1007/s11103-015-0345-7. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Yu DJ, Kim SJ, Choi D, Lee HJ. Intraspecies differences in cold hardiness, carbohydrate content and β-amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree physiol. 2012;32(12):1533–1540. doi: 10.1093/treephys/tps102. [DOI] [PubMed] [Google Scholar]
- 48.Tang Kexuan, Pan Qifang. Catharanthus roseus. Cham: Springer International Publishing; 2017. Strategies for Enhancing Alkaloids Yield in Catharanthus roseus Via Metabolic Engineering Approaches; pp. 1–16. [Google Scholar]
- 49.Schulz E, Tohge T, Zuther E, Fernie AR, Hincha DK. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci Rep. 2016;6:34027. doi: 10.1038/srep34027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee B, Henderson DA, Zhu J-K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell. 2005;17:3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva Elizamar Ciriaco, de Albuquerque Manoel Bandeira, Azevedo Neto Andre Dias de, Silva Junior Carlos Dias da. Responses of Organisms to Water Stress. 2013. Drought and Its Consequences to Plants – From Individual to Ecosystem. [Google Scholar]
- 52.Basu Supratim, Ramegowda Venkategowda, Kumar Anuj, Pereira Andy. Plant adaptation to drought stress. F1000Research. 2016;5:1554. doi: 10.12688/f1000research.7678.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gall HL, et al. Cell Wall Metabolism in Response to Abiotic Stress. Plants. 2015;4:112–166. doi: 10.3390/plants4010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavinsky AO, Magalhães PC, Ávila RG, Diniz MM, de Souza TC. Partitioning between primary and secondary metabolism of carbon allocated to roots in four maize genotypes under water deficit and its effects on productivity. Crop J. 2015;3:379–386. doi: 10.1016/j.cj.2015.04.008. [DOI] [Google Scholar]
- 55.Hochberg U, et al. Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biol. 2013;13:184. doi: 10.1186/1471-2229-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen D, Wang S, Xiong B, Cao B, Deng X. Carbon/Nitrogen imbalance associated with drought-induced leaf senescence in Sorghum bicolor. PLoS ONE. 2015;10(8):e0137026. doi: 10.1371/journal.pone.0137026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chamoli, S. & Verma, A. K. Targeting of metabolic pathways for genetic engineering to combat abiotic stress tolerance in crop plants in Approaches to plant stress and their management, 23–38 (eds Gaur, R. K. & Sharma, P.) (Springer India, 2014).
- 58.Nahar Kamrun, Hasanuzzaman Mirza, Fujita Masayuki. Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. New Delhi: Springer India; 2016. Roles of Osmolytes in Plant Adaptation to Drought and Salinity; pp. 37–68. [Google Scholar]
- 59.Ditta Allah. Molecular Stress Physiology of Plants. India: Springer India; 2013. Salt Tolerance in Cereals: Molecular Mechanisms and Applications; pp. 133–154. [Google Scholar]
- 60.Dehghan Sara, Sadeghi Mahnaz, Pöppel Anne, Fischer Rainer, Lakes‑Harlan Reinhard, Kavousi Hamid Reza, Vilcinskas Andreas, Rahnamaeian Mohammad. Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower,Carthamus tinctorius. Bioscience Reports. 2014;34(3):273–282. doi: 10.1042/BSR20140026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valifard M, Mohsenzadeh S, Niazi A, Moghadam A. Phenylalanine ammonia lyase isolation and functional analysis of phenylpropanoid pathway under salinity stress in Salvia species. AJCS. 2015;9(7):656–665. [Google Scholar]
- 62.Li S, et al. Effects of drought and salt-stresses on gene expression in Caragana korshinskii seedlings revealed by RNA-seq. BMC Genomics. 2016;7:200. doi: 10.1186/s12864-016-2562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Payyavula Raja S., Singh Rajesh K., Navarre Duroy A. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. Journal of Experimental Botany. 2013;64(16):5115–5131. doi: 10.1093/jxb/ert303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timmermann Barbara N., Steelink Cornelius, Loewus Frank A., editors. Phytochemical Adaptations to Stress. Boston, MA: Springer US; 1984. [Google Scholar]
- 65.Ramani S, Chelliah J. UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant Biol. 2007;7:761. doi: 10.1186/1471-2229-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Binder BY, Peebles CA, Shanks JV, San KY. The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol. Prog. 2009;25(3):861–5. doi: 10.1002/btpr.97. [DOI] [PubMed] [Google Scholar]
- 67.Hectors K, Prinsen E, De Coen W, Jansen MA, Guisez Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 2007;175(2):255–70. doi: 10.1111/j.1469-8137.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 68.Cantarello. C, Volpe V, Azzolin C, Bertea C. Modulation of enzyme activities and expression of genes related to primary and secondary metabolism in response to UV-B stress in Cucumber (Cucumis sativus L.) J. Plant Interact. 2007;1:151–161. doi: 10.1080/17429140600831581. [DOI] [Google Scholar]
- 69.Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14(Suppl):s185–s205. doi: 10.1105/tpc.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Cai J, Wang R, Yang S. Transcriptional regulation and transport of terpenoid indole alkaloid in Catharanthus roseus: exploration of new research directions. IJMS. 2016;18(1):53. doi: 10.3390/ijms18010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Der Fits L, Memelink J. The jasmonate‐inducible AP2/ERF‐domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate‐responsive promoter element. Plant J. 2001;25.1:43–53. doi: 10.1111/j.1365-313X.2001.00932.x. [DOI] [PubMed] [Google Scholar]
- 72.Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar K, et al. Precursor feeding studies and molecular characterization of geraniol synthase establish the limiting role of geraniol in monoterpene indolealkaloid biosynthesis in Catharanthus roseus leaves. Plant Science. 2015;239:56–66. doi: 10.1016/j.plantsci.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Doimo L, Mackay DC, Rintoul GB, D’Arcy BR, Fletcher RJ. Citronellol: geraniol ratios and temperature in geranium (Pelargonium hybrid) J. Hortic. Sci. Biotechnol. 1999;74(4):528–530. doi: 10.1080/14620316.1999.11511147. [DOI] [Google Scholar]
- 75.Singh PK, Singh R, Singh S. Cinnamic acid induced changes in reactive oxygen species scavenging enzymes and protein profile in maize (Zea mays L.) plants grown under salt stress. Physiol. Mol. Biol. Plants: Intl. J. Funct. Plant Biol. 2013;19(1):53–59. doi: 10.1007/s12298-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez-Ballesta MT, Lafuentea MT, Zacariasa L, Granellb A. Involvement of phenylalanine ammonia-lyase in the response of Fortune mandarin fruits to cold temperature. Physiol Plant. 2000;108:382–389. doi: 10.1034/j.1399-3054.2000.108004382.x. [DOI] [Google Scholar]
- 77.Janas KM, Cvikrová M, Palagiewicz A, Eder J. Alterations in phenylpropanoid content in soybean roots during low temperature acclimation. Plant Physiol. Biochem. 2000;38:587–593. doi: 10.1016/S0981-9428(00)00778-6. [DOI] [Google Scholar]
- 78.Olenichenko NA, Zagoskina NV. Response of winter wheat to cold: production of phenolic compounds and l-phenylalanine ammonia lyase activity. Appl. Biochem. Microbiol. 2005;41(6):600–603. doi: 10.1007/s10438-005-0109-2. [DOI] [PubMed] [Google Scholar]
- 79.Doll S, et al. Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant J. 2017;93(3):431–444. doi: 10.1111/tpj.13797. [DOI] [PubMed] [Google Scholar]
- 80.Cetinkaya Hakan, Kulak Muhittin, Karaman Muhammet, Karaman Halide Sedef, Kocer Ferudun. Flavonoids - From Biosynthesis to Human Health. 2017. Flavonoid Accumulation Behavior in Response to the Abiotic Stress: Can a Uniform Mechanism Be Illustrated for All Plants? [Google Scholar]
- 81.Liu Y, Meng Q, Duan X, Zhang Z, Li D. Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J Plant Interact. 2017;12(1):87–91. doi: 10.1080/17429145.2017.1293852. [DOI] [Google Scholar]
- 82.Singh-Sangwan N, Farooqi AHA, Sangwan RS. Effect of drought stress on growth and essential oil metabolism in lemongrasses. New Phytol. 1994;128:173–179. doi: 10.1111/j.1469-8137.1994.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 83.Phimchan P, Chanthai S, Bosland PW, Techawongstien S. Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in capsicum under drought stress. J Agric Food Chem. 2014;62(29):7057–62. doi: 10.1021/jf4051717. [DOI] [PubMed] [Google Scholar]
- 84.Gholizadeh A. Effects of drought on the activity of phenylalanine ammonia lyase in the leaves and roots of maize inbreds. AJBAS. 2011;5(9):952–956. [Google Scholar]
- 85.Ma D, Sun D, Wang C, Li Y, Guo T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014;80:60e66. doi: 10.1016/j.plaphy.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Mierziak J, Kostyn K, Kulma A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules. 2014;19:16240–16265. doi: 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krol A, Amarowicz R, Weidner S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiologiae Plantarum. 2014;36(6):1491–1499. doi: 10.1007/s11738-014-1526-8. [DOI] [Google Scholar]
- 88.Wang J-Y, Liu Z-P. Alkaloid Accumulation in Catharanthus roseus Increases with Addition of Seawater Salts to the Nutrient Solution. Pedosphere. 2010;20(6):718–724. doi: 10.1016/S1002-0160(10)60062-8. [DOI] [Google Scholar]
- 89.Neffati M, Sriti J, Hamdaoui G, Kchouk ME, Marzouk B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food. Chem. 2011;124:221–225. doi: 10.1016/j.foodchem.2010.06.022. [DOI] [Google Scholar]
- 90.Yan K, Cui M, Zhao S, Chen X, Tang X. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica Thunb) Front Plant Sci. 2016;7:1563. doi: 10.3389/fpls.2016.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mrázová, A. et al. Expression, activity of phenylalanine-ammonia-lyase and accumulation of phenolic compounds in Lotus japonicus under salt stress. Biologia. 72(1), 10.1515/biolog-2017-0001 (2017).
- 92.Rakhmankulova, et al. Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russ. J. Plant Physiol. 2015;62(1):71–79. doi: 10.1134/S1021443715010112. [DOI] [Google Scholar]
- 93.Abdallah S. B. et al. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiologiae Plantarum. 38(72), 10.1007/s11738-016-2096-8 (2016).
- 94.AbdElgawad H, et al. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016;7:276. doi: 10.3389/fpls.2016.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo X-R, Chang B-W, Zu Y-G, Tang Z-H. The impacts of increased nitrate supply on Catharanthus roseus growth and alkaloid accumulations under ultraviolet-B stress. J. Plant Interact. 2014;9(1):640–646. doi: 10.1080/17429145.2014.886728. [DOI] [Google Scholar]
- 96.Gil M, et al. Volatile organic compounds characterized from grapevine (Vitis vinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry. 2013;96:148–157. doi: 10.1016/j.phytochem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 97.Teklemariam TA, Blake TJ. Phenylalanine ammonia-lyase-induced freezing tolerance in jack pine (Pinus banksiana) seedlings treated with low, ambient levels of ultraviolet-B radiation. Physiologia Plantarum. 2004;122:244–253. doi: 10.1111/j.0031-9317.2004.00396.x. [DOI] [Google Scholar]
- 98.Frischknecht PM, Bättig M, Baumann TW. Effect of drought and wounding stress on indole alkaloid formation in Catharanthus roseus. Phytochemistry. 1987;26(3):707–710. doi: 10.1016/S0031-9422(00)84769-X. [DOI] [Google Scholar]
- 99.Vázquez-Flota F, Carrillo-Pech M, Minero-García Y. and De Lourdes Miranda-Ham, M. Alkaloid metabolism in wounded Catharanthus roseus seedlings. Plant Physiol Biochem. 2004;42(7–8):623–8. doi: 10.1016/j.plaphy.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 100.Liang XW, Dron M, Cramer CL, Dixon RA, Lamb CJ. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989;264(24):14486–92. [PubMed] [Google Scholar]
- 101.Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in. Arabidopsis. Plant Physiol. 2005;140(2):637–46. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh DV, Maithy A, Verma RK, Gupta MM, Kumar S. Simultaneous determination of catharanthus alkaloids using reversed phase high performance liquid chromatography. J. Liquid Chromatogr. Related Tech. 2000;23(4):601–607. doi: 10.1081/JLC-100101476. [DOI] [Google Scholar]
- 103.Gupta MM, et al. Simultaneous Determination of Vincristine, Vinblastine, Catharanthine, and Vindoline in Leaves of Catharanthus roseus by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2005;43(9):450–453. doi: 10.1093/chromsci/43.9.450. [DOI] [PubMed] [Google Scholar]
- 104.Amthor JS. Efficiency of LigninBiosynthesis: a Quantitative Analysis. Annals Bot. 2003;91:673–695. doi: 10.1093/aob/mcg07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dutta A, Sen J, Deswal R. Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G. Don. Plant Cell Rep. 2007;26(10):1869–78. doi: 10.1007/s00299-007-0383-y. [DOI] [PubMed] [Google Scholar]
- 106.Vom Endt D, Silva MSE, Kijne JW, Pasquali G, Memelink J. Identification of a bipartite jasmonate- responsive promoter element in the Catharanthus roseus ORCA3 transcription factor gene that interacts specifically with AT-Hook DNA-binding proteins. Plant Physiol. 2007;144(3):1680–1689. doi: 10.1104/pp.107.096115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruijter JM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin Z, et al. Simultaneous quantitative determination of five alkaloids in Catharanthus roseus by HPLC-ESI-MS/MS. Chin J Nat Med. 2014;12(10):786–793. doi: 10.1016/S1875-5364(14)60120-5. [DOI] [PubMed] [Google Scholar]
- 110.Chou K-C, Shen H-B. Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE. 2010;5:e11335. doi: 10.1371/journal.pone.0011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu J, et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khang Chang Hyun, Park Sook-Young, Rho Hee-Sool, Lee Yong-Hwan, Kang Seogchan. Agrobacterium Protocols Volume 2. Totowa, NJ: Humana Press; 2006. Filamentous Fungi (Magnaporthe grisea and Fusarium oxysporum) pp. 403–420. [DOI] [PubMed] [Google Scholar]
- 113.Ortega Jose Luis, Wilson Olivia L., Sengupta-Gopalan Champa. The 5′ untranslated region of the soybean cytosolic glutamine synthetase β1 gene contains prokaryotic translation initiation signals and acts as a translational enhancer in plants. Molecular Genetics and Genomics. 2012;287(11-12):881–893. doi: 10.1007/s00438-012-0724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu D, et al. Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE. 2012;7(9):e46451. doi: 10.1371/journal.pone.0046451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomlinson KL, et al. Evidence that the hexose-to-sucrose ratio does not control the switch to storage product accumulation in oilseeds: analysis of tobacco seed development and effects of overexpressing apoplastic invertase. J Exp Bot. 2004;55(406):2291–303. doi: 10.1093/jxb/erh251. [DOI] [PubMed] [Google Scholar]
- 116.Bradford MM. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.