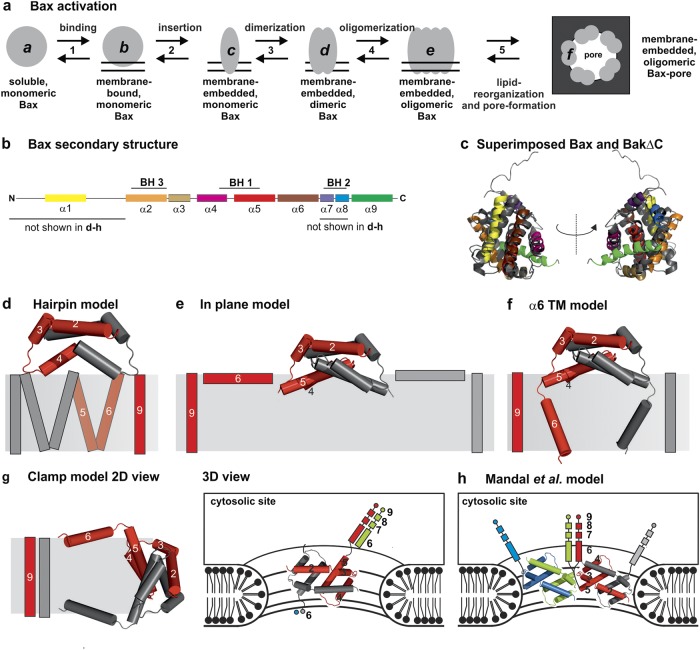
Fig. 1.
Topology models of Bax and Bak. a Sketch of the conformational changes of Bax during the transformation from the soluble, monomeric, inactive state to the membrane-embedded, oligomeric, pore-forming state. The protein can exist at least in six conformational states (a–f). b Schematic view of the secondary structure of Bax with the BH domains highlighted. c Superposition of the structures of soluble, monomeric Bax [15] (color code as in b) and BakΔNΔC (dark gray) [16]. d–g 2D representation of four published topology models of membrane-embedded Bax/Bak dimers. One monomer is shown in gray and the other in red. The structure of helices 2–5 is based on the PDB: 4BDU [25]. d Hairpin model based on Annis et al. [49]. e “In-plane” model as proposed by Westphal et al. [47]. f, g Models of Bax based on a structural model suggested by Bleicken et al. [30]: model considering a planar bilayer (panel f, α6 TM model) and the “clamp” model in the context of a toroidal pore (panel g, left: 2D model, right: 3D model with possible contacts to other helices 6–9 of adjacent dimers in green and blue). h Model suggested by Mandal et al. [50]. Two dimers are shown, one in gray and red, and the second in blue and green