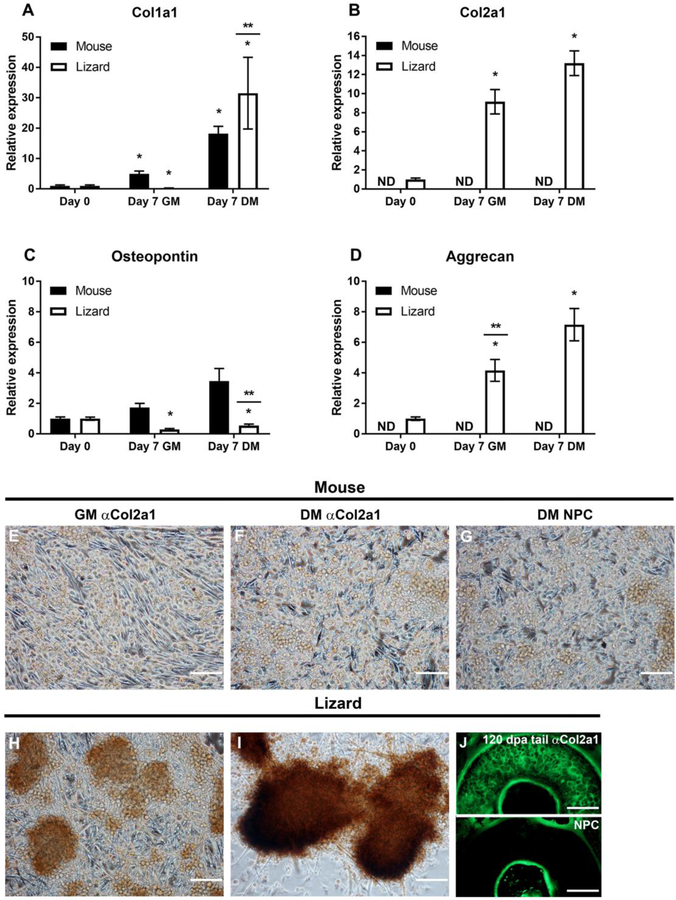
Figure 6. Extracellular matrix gene expression in micromass cultures demonstrate that lizard cells express cartilage-specific genes.
Micromasses were cultured for 7 days in growth (GM) or chondrogenic differentiation media (DM). Gene expression was determined by gene specific qRT-PCR. Collagen 1a1 (Col1a1), was up-regulated in both species in DM (A), while collagen 2a1 (col2a1), a cartilage specific collagen, was detected in lizard satellite cells only (B). Osteopontin (Opn), was increased in mouse cells but not significantly, it was significantly down-regulated in lizard cells (C). Aggrecan (acan) expression was detected only in lizard micromasses, where it was significantly up-regulated in GM and further increased in DM (D). qRT-PCR data are expressed as relative gene expression and are the result of 3 biological replicates, each done in triplicate. Statistical analysis was done by one way ANOVA; * indicates p<0.05 when compared to Day 0 and ** denotes that DM and GM for that species are significantly different from each other (p<0.05); ND= not detected. Protein expression was determined by ICC, using a monoclonal anti-COL2A1 antibody and anti-mouse-HRP and the DAB substrate to detect the protein. Mouse satellite cells exhibit background staining that is indistinguishable from the no primary antibody control (NPC) sample (E-G). Chondrogenic nodules express COL2A1 protein in lizard micromasses in both culture conditions, although the staining is more robust in DM (H, I). To show the specificity of the anti-COL2A1 antibody in lizard, IF was done with an anti-mouse-FITC secondary antibody on transverse sections of regenerating lizard tails 120 DPA. The matrix of the cartilage is evident compared to the NPC control (J).