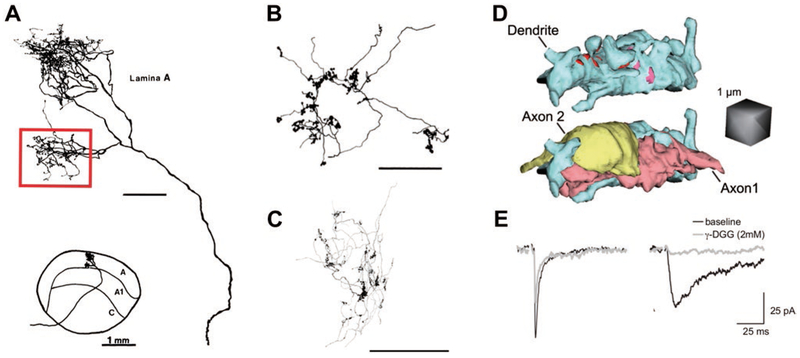
Fig. 1.
Synaptic structure shapes retinogeniculate transmission. (A) Tracing of an HRP-filled X-RGC arbor in the cat dLGN shows the location and morphology of a single branch (red box) of the X-RGC arbor used for EM reconstruction. This branch of the axon contacts 4 TC neurons out of 40 available neurons in the territory of the arbor. The remainder of the axon was not reconstructed, and likely contacts several other TC neurons. Bottom inset shows the location of the axonal arbor in the context of the cat LGN. Figure modified from Hamos et al. (1987). Unmarked scale bar = 100 μm. (B, C) Reconstructed arbors of single RGC axons showing distribution of presynaptic boutons into dense clusters in the LGN of (B) an adult cat and (C) a p20 mouse. Note the clustering of boutons along the arbor. Image in B is modified from Robson et al. (1993), showing a segment of a RGC axon; Image in C is from Hong et al. (2014), showing a BD-RGC axon. Scales bars are 100 μm. (D) A 3D reconstruction of a TC neuron dendrite and sites of contact between two neighboring RGC boutons from Budisantoso et al. (2012). In the top image, the dendrite and its appendages are depicted in blue, whereas pink and red sites label the postsynaptic densities of the two axons. In the bottom image, the structure of the terminals of two axons has been added. Spillover can occur between these two nearby terminals. (E) Evidence of spillover-mediated responses to the stimulation of a single RGC axon before eye opening. Two different synaptic responses were observed in response to single retinal fiber stimulation. Shown are recordings from TC neurons in whole cell voltage clamp at −70 mV in a dLGN slice in the presence of the NMDAR blocker, 20 μM CPP. On the left is an example of a retinogeniculate AMPAR EPSC with characteristic rapid rise time and decay kinetics (black trace). On the right is an atypical AMPAR EPSC response notable for significantly slower rise time and decay kinetics (black trace). The two types of EPSCs differ in their sensitivity to the low-affinity AMPAR antagonist, γ−DGG. Low affinity antagonists can be used to assess the relative concentration of glutamate in the synaptic cleft (Clements et al., 1992; Diamond & Jahr, 1997). As γ−DGG competes with glutamate for binding to AMPAR, its efficacy of inhibition decreases with increasing glutamate concentration. γ−DGG has only a small effect on the amplitude of the fast EPSC, but dramatically reduces the amplitude of the slow EPSC (overlaid gray traces), consistent with lower peak glutamate concentration in the synaptic cleft of the slow EPSC. Because the EPSCs are evoked by minimal stimulation, the rapid EPSC represents a direct input from a single RGC axon that forms a direct synapse onto the voltage-clamped relay neuron, whereas the slow EPSC corresponds to the activation of a RGC axon that does not directly synapse onto the voltage-clamped neuron. Modified from Hauser et al. (2014). All figures reprinted with permission.