Abstract
Anthropogenic use of high density, toxic elements results in marine pollution which is bio-accumulating throughout marine food webs. While there have been several studies in various locations analyzing such elements in fish, few have investigated patterns in these elements and their isotopes in terms of ocean depth, and none have studied the greatest depth zones. We used a flame atomic absorption spectrophotometer-hydride system and an inductively coupled plasma-mass spectrometer to determine concentrations of the high-density elements arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), selenium (Se), plus the light-metal barium (Ba), in fish ranging from bathyal (1000 m in Monterey Bay) to upper hadal zones (6500–7626 m in the Kermadec and Mariana Trenches) in the Pacific Ocean. Five species of fish—including the Mariana Trench snailfish, the world's deepest known fish newly discovered—were analyzed for patterns in total element concentration, depth of occurrence, Se:Hg ratio, plus mercury isotopes in the deepest species. Co and As levels decreased with depth. In the Mariana Trench, Pb, Hg, Cd, and Cu were higher than in all other samples, and higher in those plus Ba than in the Kermadec Trench. The latter samples had far higher Ni and Cr levels than all others. Mercury relative isotope analysis showed no depth trends in the deepest species. Se:Hg showed a large molar excess of Se in bathyal flatfish species. These patterns indicate that exposures to pollutants differ greatly between habitats including trenches of similar depths.
Keywords: Environmental science, Geochemistry, Oceanography, Earth sciences, Biogeoscience
1. Introduction
Numerous naturally occurring high density toxic elements have significantly bio-concentrated in coastal aquatic ecosystems due to anthropogenic activity (Burger et al., 2014). The anthropogenic input of these elements into fish can be traced back to different sources including mining and smelting, industrial processing, breakdown of sediment by acidic rain, outdated infrastructure, pesticides/herbicides and countless more (Tchounwou et al., 2012). It is important to trace the movement and bioaccumulation of these elements because they can directly affect aquatic species and can be transferred up the food chain to humans, causing multiple organ damage (Burger et al., 2006).
Mercury (Hg) in particular has received considerable attention, especially in the form of monomethylmercury (CH3Hg+) which readily bio-accumulates and is thought to be the primary toxic form entering humans (Burger et al., 2006; Bergquist and Blum, 2007). Several studies have been done analyzing the concentrations of CH3Hg+ in freshwater fish tissue, but marine ecosystems are more difficult to source given the migration of water, contaminants and atmospheric deposition (Fitzgerald et al., 2007). Anoxic conditions induced by anthropogenic activity such as reservoir construction and sulfate-loading have been shown to expedite the methylation of inorganic Hg, which can be carried into open oceans (Scudder et al., 2009). The three main regions of methylation in marine systems are coastal sediments, low oxygen waters, and deep water sediments while the four main mechanisms are the reduction of Hg(II), degradation of CH3Hg(I), methylation of inorganic Hg and the oxidation of Hg(0) (Gworek et al., 2016). The sulfate and iron reducing mechanisms of coastal sediments by bacteria as well as the trophic transfer of surface plankton to the base of the food web are better understood than the mechanisms that deep water sediment methylation undergo (Slowey and Brown, 2007). This means that anthropogenic Hg sources are easier to track in the surface level (0–100 m) and get blurred in the water-mixing region (100–1000 m) and therefore even more difficult in sub-thermocline regions. Microbial reactions in fish can also transform inorganic Hg isotopes into CH3Hg+ (Blum et al., 2013). Choy et al. (2009) determined that these processes create vertical profile concentrations in which deeper aquatic species (e.g., tuna, swordfish, mackerel, and sharks) contain higher concentrations of mercury than surface level fish in open waters due to a sub-thermocline low-O2 environment.
Stable isotopes of Hg (which are 196, 198, 199, 200, 201, 202 and 204 amu) can also accumulate differentially. Biotic and abiotic reactions in the fish induce mass-dependent (isotope) fractionation (MDF, expressed as δ202Hg) of CH3Hg+ while photochemical processes can induce mass-independent fractionation (MIF, expressed as Δ199Hg, Δ201Hg) (Bergquist and Blum, 2007). Delta notation reflects relative deviation from the average isotopic makeup of a given element. On the aggregate, by averaging a large sample size, these delta values could be used to trace the origin of different species of Hg in sample tissue, as different sources of Hg will have a different relative deviation from the standard isotopic makeup.
Mercury has been intensively studied in fish because of its prevalence, biogeochemical transformation pathways in metabolic processes, and toxicity; but there are other essential and nonessential high-density elements that have been found to be toxic based on their molar speciation that have not been studied extensively in fish (Perrot et al., 2010). Chromium and copper are examples of essential metals in plants and animals that use the reduction process to activate cuproenzymes (Stern, 2010). However, when copper is overly concentrated, the reduction of Cu[II] to Cu[I] can produce superoxide and hydroxyl radicals which induce cellular and tissue damage (ATSDR, 2004). There is an extremely narrow range in which these metals are beneficial for the animal or plant before they become toxic, and for non-essential high density elements (e.g., As and Pb), just trace amounts (ppt-ppb) can be toxic (Chang et al., 1996). Studying the fractionation of these metals can provide an understanding of the biogeochemical cycles that have already been discovered for mercury.
Once there is enough known about the fractionation processes of these high density elements, source and process tracing can be used to quantify and locate the presence of the isotopes deemed harmful (Blum and Erel, 2003). By determining the isotopic compositions of certain locations prior to the addition of source metals, the quantity of anthropogenic or natural contributions can be determined (Wiederhold, 2015). This can be done sufficiently using known literature metal concentration values of a certain species of fish before a known pollution event and comparing it to concentration levels of the fish afterwards (Chen et al., 2008). For example, if literature delta values of Pacific ocean fish showed 0.5‰ for barium in 1990, and throughout the decade coal sludge with a −1.5‰ value was being polluted into the Pacific, a delta value of −1.0‰ in the fish in 2000 would indicate an anthropogenic contribution of 75% of barium over 10 years (Sun et al., 2014). By determining isotope delta values at know pollution sites, the source of the metal concentration in the fish can be traced. The main issue with this technique is that there are multiple sources that contribute to the bioaccumulation of these metals in fish and it is difficult to pinpoint the source without a very large sample size (Estrade et al., 2010).
However, by analyzing fish that inhabit varying depth levels, conclusions can be made about the source of high-density element pollution (Larsson et al., 2007). Marine producers that inhabit the epipelagic level are at the bottom of the aquatic food chain, and they absorb a variety of such elements that can be traced back to the nearest industrial area if they are in close proximity to land (Bashir et al., 2012). Once the isotopic compositions of these species are known they can be tracked up the food chain to larger species through an understanding of microbial reactions in the metabolic processes of different species of fish (Perrot et al., 2010). An established trend of high-density element concentration based on depth of inhabitants can provide insight into the bioaccumulation process as well as a distinction between anthropogenic versus natural pollution (Chen et al., 2000).
This study attempts to better understand the bio-accumulation of high density, toxic elements (plus barium) in fish inhabiting varying depth levels of the Pacific Ocean. This involves analysis of total high-density element concentrations of fish that inhabit depth zones ranging from the surface of the ocean to the deepest trenches (Albare'de et al., 2004). Specifically we determined concentrations for arsenic (As), cadmium (Cd), barium (Ba, the only low-density metal), nickel (Ni), copper (Cu), cobalt (Co), lead (Pb), selenium (Se), chromium (Cr), and mercury (Hg), in 5 different species of fish ranging from 1,000 m (bathyal zone, Monterey Bay, California) to 7,626 m (hadal zone in tectonic trenches). The trench species are the Kermadec snailfish, recently rediscovered after a 50-year hiatus, and the newly discovered Mariana Trench snailfish, the world's deepest known vertebrate (Linley et al., 2016; Gerringer et al., 2017a); both are very difficult to obtain.
Using these values and published values for a shallow fish of the Aleutians (Burger et al., 2014), we examined the hypothesis that high-density element concentrations in fish vary as a function of depth of occurrence in the water column. We also analyzed isotopic compositions for Hg in the deepest species. By comparing these values to previous literature data, sediment core concentrations, and coal concentrations, their origin can potentially be uncovered.
2. Materials and methods
2.1. Sample collection and preparation
Fish were captured and processed according to our protocol 0214R approved by Whitman College's Institutional Animal Care and Use Committee.
Deep fish species were acquired by the University of Hawaii's hadal fish trap on the R/Vs Thompson and Falkor in 2014 (Linley et al., 2016). These were the abyssal grenadier Coryphaenoides armatus near the rim of the Kermadec Trench at 5000 m, and the hadal (trench) liparid or snailfishes at 7500–7626 m: Notoliparis kermadecensis (Kermadec Trench) and a new species Pseudoliparis swirei in the Mariana Trench, the world's deepest known fish (Gerringer et al., 2017a).
Flatfish species—Dover Sole, Microstomus pacificus and Deep Sea Sole, Embassichthys bathybius—were acquired by the NOAA's U.S. West Coast Groundfish Bottom Trawl Survey, at a depth of 1,000 m in Monterey Bay, California, in 2010.
Fish were kept frozen at −80 °C. White muscle tissue samples were acquired from the dorsal region of the fish and were digested in concentrated nitric and concentrated sulfuric acid: for flatfish, in 1.5 mL of nitric acid and 4.0 mL of sulfuric acid for ≈.5 g of tissue; for trench fish in 0.01 mL of nitric acid and 0.04 mL of sulfuric acid for ≈0.05 g of tissue. All samples were allowed to digest for 48 hours before being diluted to 100 mL (flatfish) or 25.0 mL (trench fish) with ultrapure deionized water.
2.2. Chemicals used in analysis
All standards were prepared using commercially available atomic absorption 1000 mg/L metal standards (RICAA chemical company) with at least 1% ultra-pure nitric acid to preserve the standards. The mercury standard used was a NIST standard (SRM-3133) with reported isotopic ratios.
The acids used in the study were double-distilled concentrated nitric acid, concentrated sulfuric and concentrated HCl acid (ultra-pure Aristar Plus, VWR Scientific Products). The water used was doubly deionized to make it ultra-pure (<18.2 MΩ cm at 25 °C) (Millipore Milli-Q system). Prepared samples and calibration curve standards were stored in 150 mL polypropylene bottles. Polypropylene is used to minimize metal adsorption onto polar glass surfaces to increase the aqueous metal ions in solution which would in turn preserve the original metals in each sample.
Blanks were prepared with the same amount of acid as the dilute fish digestions, 1.5% nitric and 4% sulfuric, before any adaption was made for specific techniques. All standards were prepared using Eppendorf pipets for accuracy when diluting. All samples were filtered with 0.4 - micron syringe filters (VWR number 28145-493) before being introduced into the instruments.
2.3. Element analysis
An Agilent 7500ce Inductively Coupled – Mass Spectrometer (ICP-MS) was used to determine the trace metal content in the fish samples. Metals analyzed include chromium, nickel, cobalt, copper, zinc, cadmium, silver, barium, mercury, and lead. Metal analysis was performed using external calibration with standards ranging from 1 ng/L to 250 mg/L. Calibration was done using the instrument's software. Each calibration standard was run with 1% ultrapure nitric acid. Internal standards of 50 mg/L bismuth (Bi), indium (In), and gallium (Ga) were used to decrease detection limits and monitor quadrupole tuning drift. The parameters used for the ICP-MS are found in Appendix A. The NIST Hg isotope standard noted above was used for calculation of isotope composition.
Selenium (80Se) has a major isobaric interference with a major argon-argon dimer when analyzed by ICP-MS; to determine the selenium content in the samples accurately we used a hydrogen gas reaction cell with the ICP-MS. Analysis of selenium was performed using an external calibration with standards ranging from 100 ng/L to 250 mg/L. Each calibration standard was prepared with 1% nitric acid and 1% methanol. The parameters used when running the ICP-MS with the reaction cell are found in Appendix B. Arsenic was analyzed on a Perkin Elmer 400 Flame Atomic Absorption Spectrometer (FAAS) using the MHS 115 (Mercury Hydride System) attachment. The arsenic hydride attachment (the same as the MHS) was used because it greatly improves the detection limit using the FAAS. The reducing agent used was 3% NaBH4 and 1% NaOH in Milli-Q water. The determination of arsenic was performed using external calibration with standards from 100 ng/L to 100 mg/L. The standards were run with 1% nitric acid. To each 10.0 mL standard and sample 2.00 mL of L-cysteine pre-reduction solution was added. The pre-reduction solution was prepared with 5.00 g of L-cysteine in 100 mL of 5% HCl solution. The standards and samples were allowed to stand for 30 minutes before running. Pre-reduction is necessary because the hydride is generated much more slowly from As(V) than As(III), and so pre-reduction increases the detection limit and accuracy of sample measurement (Welz and Šucmanová, 1993). The fish samples foamed aggressively, making them difficult to analyze, and 1 mL of antifoaming agent was added to each sample to reduce the foaming (Xiameter AFE-0110 Antifoam Emulsion).
2.4. Statistics
For each element, one-way ANOVA tests were run on all groups except the Kermadec grenadier (n = 1). Tukey post-hoc tests were then conducted between each group. Significance was set at P < 0.05.
3. Results
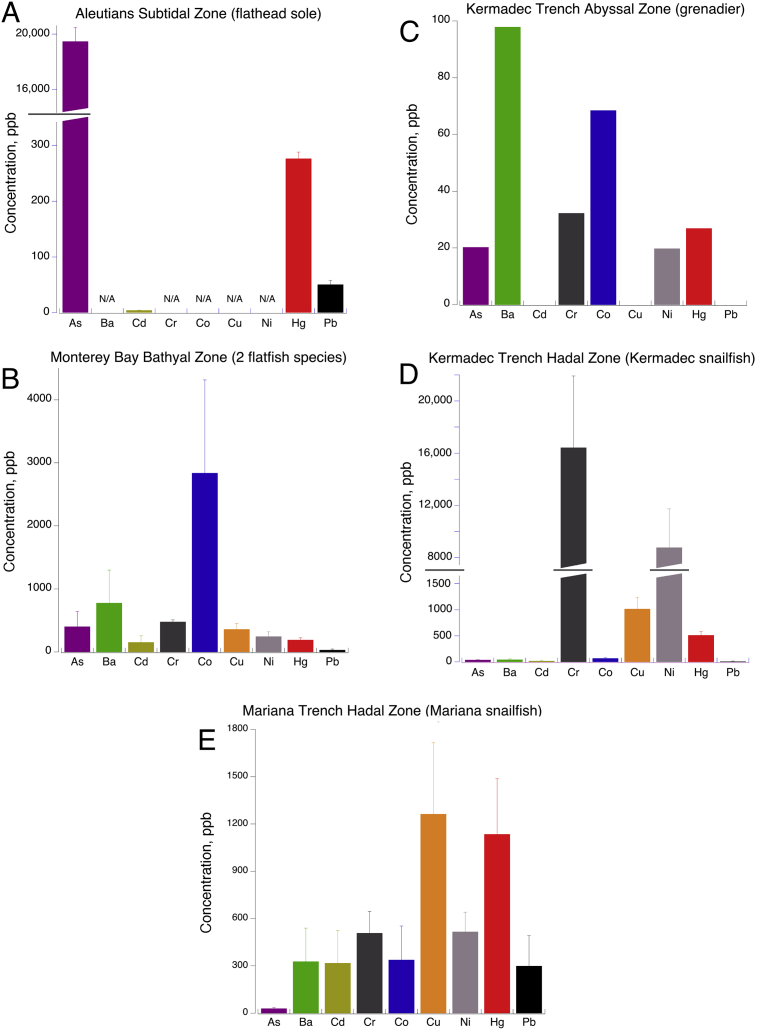
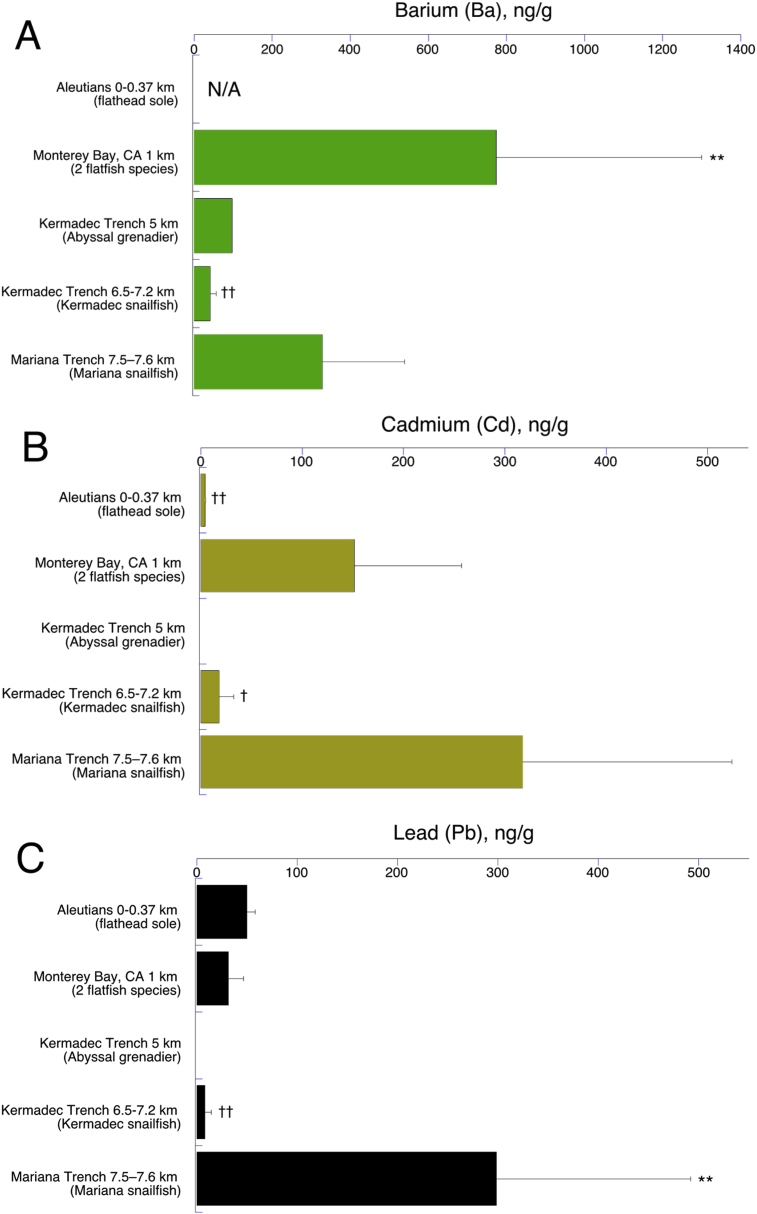
Our data, in addition to those of a flatfish from the Aleutians from a previously published study (Burger et al., 2014), showed considerable differences in the levels of high density elemental concentrations in fish muscle. In some cases these may relate to depth of capture, in other cases to local habitat features, or both (Table 1). A summary of elements by location is shown in Fig. 1. The patterns for our 4 locations differ widely. The single fish from the Kermadec abyssal zone had overall the lowest concentrations (Fig. 1C; compare its ordinate to those of Fig. 1A, B, D, E); the Kermadec Trench snailfish (Fig. 1D) had extremely high levels of nickel (Ni) and chromium (Cr), while the Mariana Trench snailfish had the most of these toxic elements at considerable levels (all but arsenic As; Fig. 1E).
Table 1.
High-density element concentrations (plus barium) in ppb (ng/g, wet weight) of five species collected from four different locations for this study plus one species from Aleutians [flathead sole (Hippoglossoides elassodon)] taken from Burger et al. (2014), shown as means ± standard error and detection limits of each element. N/A = not analyzed; **indicates statistically higher than all other groups (P < 0.001 except for Ba where P < 0.05); *statistically higher than other groups but lower than **group (P < 0.05); ††statistically lower than all other groups (P < 0.05); †statistically lower than other groups but higher than ††group (P < 0.05).
| Aleutians Subtidal zone (1 flatfish species) |
California Bathyal zone (2 flatfish species) |
Kermadec Abyssal zone (Abyssal grenadier) |
Kermadec Trench Hadal zone (Kermadec snailfish) |
Mariana Trench Hadal zone (Mariana snailfish) |
Detection Limit | |
|---|---|---|---|---|---|---|
| 0–366 m |
1000 m |
5,000 m |
6,500–7,250 m |
7509–7626 m |
||
| n = 39 | n = 6 | n = 1 | n = 7 | n = 5 | ||
| Arsenic | 19,500 ± 1013** | 403.0 ± 236* | 20.2 | 35.17 ± 4.72 | 28.22 ± 7.23 | 2.00 |
| Chromium | N/A | 475.0 ± 34.1 | 32.20 | 16,400 ± 5500** | 508.3 ± 138.2 | 20.0 |
| Nickel | N/A | 244.2 ± 76.5 | 19.80 | 8766 ± 2974** | 516.3 ± 123.6 | 24,000 |
| Cobalt | N/A | 2834 ± 1479** | 68.45 | 65.97 ± 18.0 | 336.9 ± 215.3 | 24.0 |
| Copper | N/A | 360.4 ± 90.7†† | 0.00 | 1013.8 ± 222.6 | 1262 ± 454 | 200 |
| Cadmium | 4.2 ± 0.5†† | 151.6 ± 105.6 | 0.00 | 18.03 ± 14.4† | 317.4 ± 206.8 | 0.045 |
| Barium | N/A | 773.8 ± 526.5** | 97.83 | 40.89 ± 15.3†† | 328.2 ± 210.8 | 2.50 |
| Mercury | 276 ± 12 | 189.9 ± 33.5 | 26.93 | 507.5 ± 71.9* | 1135 ± 353** | 4.00 |
| Lead | 50.3 ± 8.0 | 31.31 ± 15.3 | 0.00 | 7.96 ± 6.39†† | 298.4 ± 193.7** | 0.05 |
| Selenium | 398 ± 37.4 | 931.1 | N/A | N/A | N/A | 500 |
Fig. 1.
High-density element concentrations (plus barium) in fish muscle from each sample collection site. Error bars are standard errors. Note the very large ordinate scale change for As in panel A for the Aleutian study (Burger et al., 2014; N/A = not analyzed in that study), and the large scale change for Cr and Ni in panel D.
3.1. Trends by depth and habitat
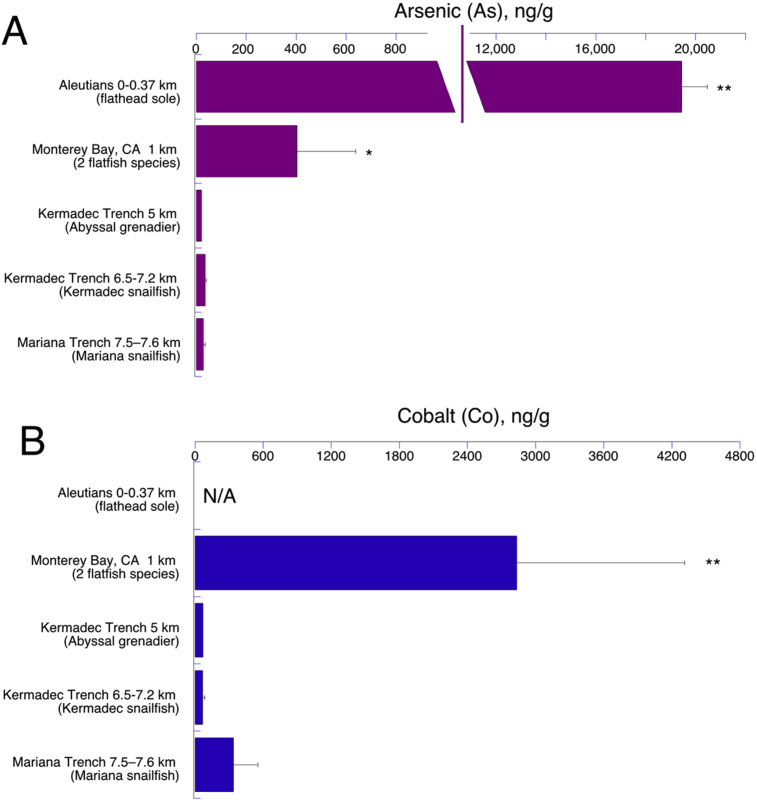
Analytes arsenic (As), cobalt (Co), copper (Cu), and mercury (Hg) showed apparent trends with depth especially with regard to the trenches (Figs. 2 and 3). Arsenic contents were lower at greater depths: the Aleutian flatfish and our bathyal (1 km) flatfish had the highest values (Table 1; Fig. 2A). The Aleutian study included two other flatfish species, and those too had much higher arsenic than our samples (not plotted; Burger et al., 2014). Cobalt showed a similar pattern in our samples in that the bathyal (1 km) flatfish had by far the highest concentration (Fig. 2B; no data available for Aleutian fish).
Fig. 2.
Concentrations of Arsenic (A) and Cobalt (B) in fish muscle by collection depth (with deepest specimens at bottom left of plot). Note the very large scale change for ng/g in panel A. N/A = not analyzed in the Aleutian study (Burger et al., 2014). Error bars are standard errors; **indicates statistically higher than all other groups (P < 0.001); *statistically higher than other groups but lower than **group (P < 0.05).
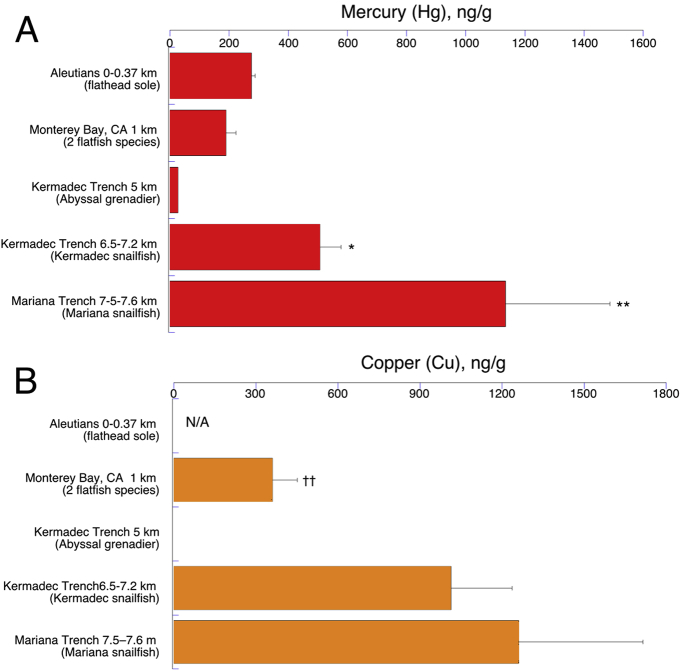
Fig. 3.
Concentrations of Mercury (A) and Copper (B) by collection depth as in Fig. 2. N/A = not analyzed in Aleutian study (Burger et al., 2014). Error bars are standard errors; *, ** indicate statistics as in Fig. 2; †† indicates statistically lower than all other groups (P < 0.05).
Mercury, in contrast showed moderate levels (190–276 ppb) in the flatfish groups rising to much higher contents in the trench snailfish groups, reaching 1134 ppb in the Mariana specimens (Fig. 3A). Copper was similarly highest in the trenches, increasing from 360 to 1262 ppb in our samples (Fig. 3B; no data available for Aleutian fish). These high density elements were lowest in the abyssal Kermadec grenadier, but with only 1 specimen available, statistical comparisons are not possible.
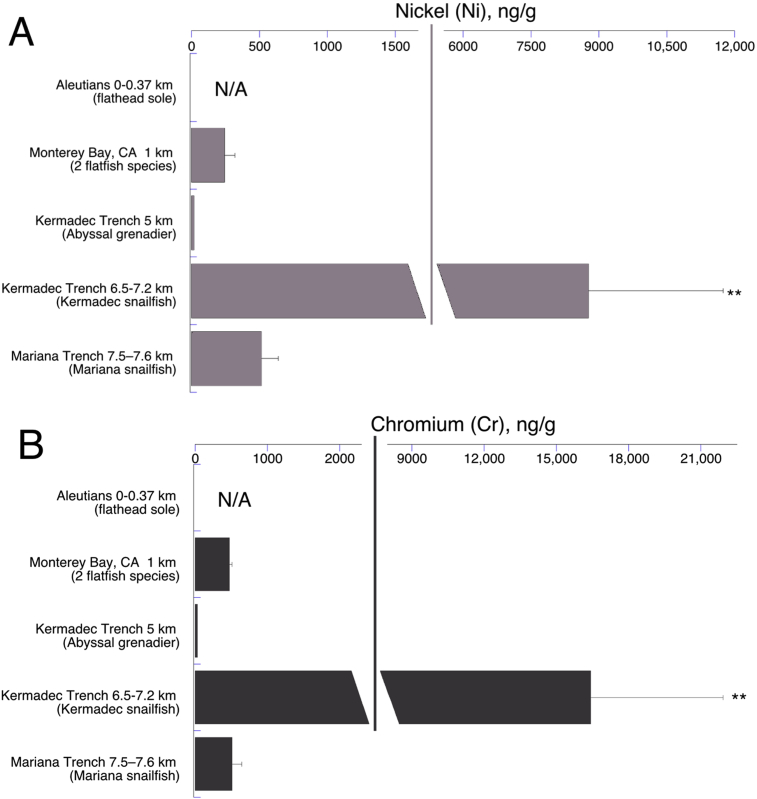
Nickel (Ni) and chromium (Cr) showed a large spike in concentration in the Kermadec Trench snailfish, captured at 6.5–7.2 km, relative to the shallower fish and the snailfish of same zone (hadal) in the Mariana Trench (Fig. 4A and B). Though those Mariana snailfish were slightly deeper (7.5–7.6 km), it is at the same trophic level as the Kermadec snailfish—both are carnivore/scavengers whose dominant food items are live scavenging amphipods (Gerringer et al., 2017b), so it is more likely that the trench samples differ due to geographical location rather than depth. The extremely high Ni and Cr contents in the Kermadec Trench fish were particularly unexpected (see section 4.1 Discussion).
Fig. 4.
Concentrations of Nickel (A) and Chromium (B) by collection depth as in Fig. 2. N/A = not analyzed in Aleutian study (Burger et al., 2014). Note the large scale change for ng/g in both panels. Error bars are standard errors; **indicates statistics as in Fig. 2.
The remaining metals – cadmium (Cd), barium (Ba), and lead (Pb)—had more complex patterns. Ba was lowest in the Kermadec Trench fish, and highest in Monterey Bay bathyal flatfish at 1 km, with the Mariana Trench fish being second (Fig. 5A). Cd was lowest in the Aleutian and Kermadec Trench fish and highest at in the Monterey Bay and Mariana Trench fish (Fig. 5B; those two groups did not differ statistically from each other). Pb trended lower with depth from the Aleutian to the Kermadec Trench fish (in which it was lowest), but then showed the highest value by far in the Mariana Trench fish (Fig. 5C).
Fig. 5.
Concentrations of Barium (A), Cadmium (B), and Lead (C) by collection depth as in Fig. 2. N/A = not analyzed in Aleutian study (Burger et al., 2014). Error bars are standard errors; **, †, †† indicate statistics as in Fig. 2 except for barium (panel A) where ** indicates P = 0.05.
3.2. Se:Hg, MDF, delta values and Hg relative isotope analysis
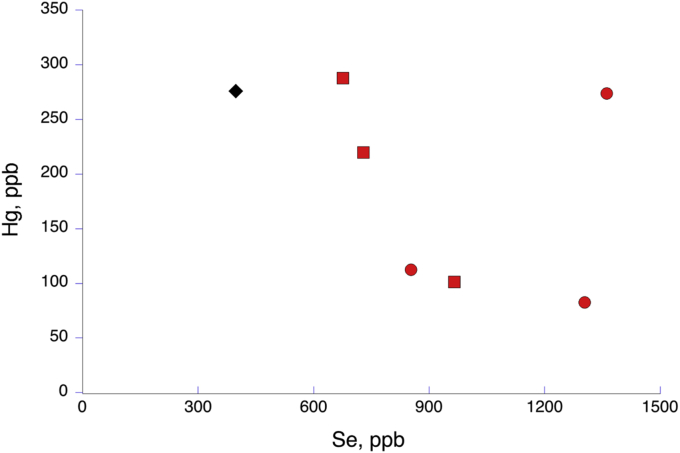
Selenium:mercury ratios, measured only in the Monterey Bay fish in our study, showed that Se was present in much higher concentrations. In these coastal fish, that ratio varied from 6:1 to 40:1, all statistically higher than in the Aleutian flatfish at 1.44:1 (Table 2; Fig. 6).
Table 2.
Se:Hg molar ratio in flatfish caught from 1000 m off of the coast of California (n = 5 muscle samples for each fish, with standard errors), plus in a flatfish from the Aleutian study (n = 39; Burger et al., 2014). Dover sole 2 had a higher value than all others (*; p < 0.02) except compared to Deep-sea sole 2 (p = 0.15), while the flathead sole value was statistically lower (†; p < 0.001) than all of our samples.
| Dover Sole 1 | Dover Sole 2 | Dover Sole 3 | Deep-sea Sole 1 | Deep-sea Sole 2 | Deep-sea Sole 3 | Aleutians Flathead Sole |
|---|---|---|---|---|---|---|
| 12.6 ± 4 | 40 ± 15* | 19 ± 4 | 8 ± 2 | 24 ± 6 | 6.0 ± 0.5 | 1.44 ± 0.1† |
Fig. 6.
Relationship between total Se concentration and total Hg concentration in subtidal and bathyal flatfish. Red circles are Dover Sole; red squares are the Deep Sea Sole; the diamond is the flatfish value from the Aleutian study (Burger et al., 2014).
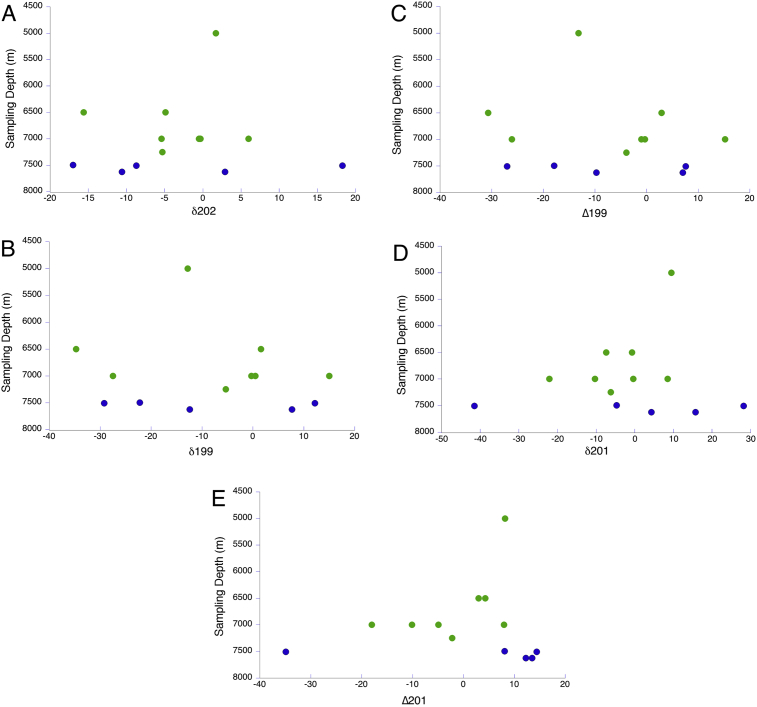
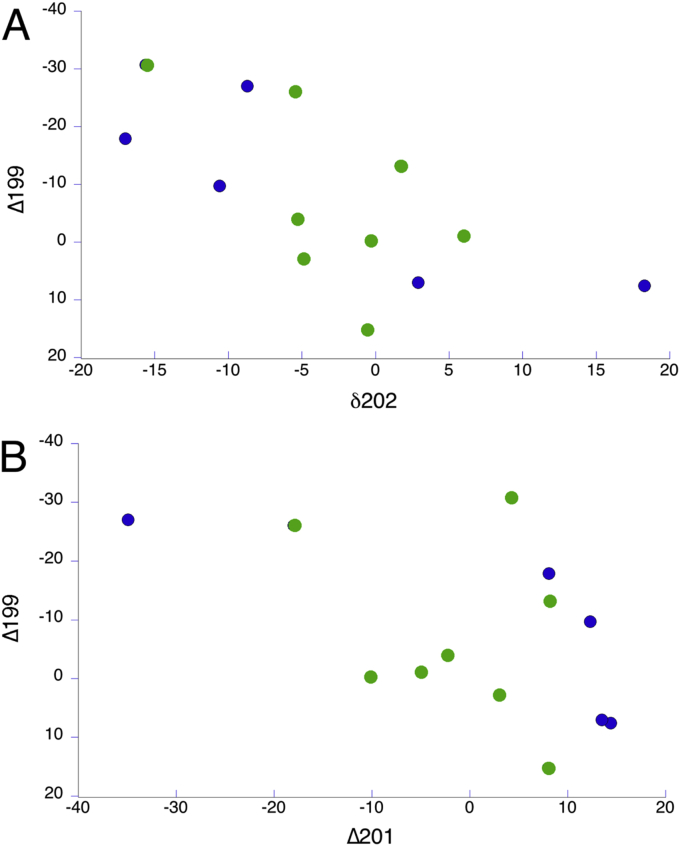
The ratios used in analysis, in delta notation, are Δ199Hg, Δ201Hg (MIF), and δ202Hg (MDF) (see Introduction). Experimentally determined values were compared to the NIST standard. The experimentally determined ratios of calibration standards stayed consistent through the lowest concentration standards, statistical analysis indicating no bias (P = 0.05). This means that even samples that fall below the detection limit of the external calibration give statistically significant information from isotopic ratio analysis of Hg. The grenadier and snailfish from the Kermadec and Mariana trenches, which exhibited relatively high concentrations of Hg, were the primary focus of isotope analysis. MDF as δ202 Hg and MIF as △199Hg, △201Hg delta values, were calculated for these fish, which ranged from 5,000 m to 7,626 m (Figs. 7 and 8). There is no clear trend in any of the relationships of relative ratios and depth or MIF and MDF.
Fig. 7.
Relative mercury isotope ratios in delta notation (see Introduction) plotted with depth of samples for Δ199 Hg (A), δ199 Hg (B), δ201 Hg (C), δ202 Hg (D) and Δ201Hg (E). The green circles are samples from the Kermadec Trench, the blue circles from the Mariana Trench.
Fig. 8.
Relationships between mercury relative isotope ratios in delta notation (see Introduction) for Δ199 vs δ202 Hg (A) and Δ201 Hg (B). The green circles are samples from the Kermadec Trench, the blue circles from the Mariana Trench.
4. Discussion
In this study, fish from bathyal to hadal zones were analyzed for high density, toxic elemental content, including the deepest fish ever encountered. While our sample sizes were small, and a wide variation of high density, toxic elemental content and isotopic ratios showed trends that indicate that further research on toxic high-density elements and ocean depths is warranted.
4.1. Depth and habitat patterns
Toxic high-density elemental concentrations in fish in this study varied widely. High concentrations of most high density elements were found in the fish inhabiting the Mariana and the Kermadec trenches, indicating that deep trenches may be sites where pollutants can accumulate. Recent modeling suggests that even remote trenches may act like "funnels" and collect pollutants (as well as nutrients) from distant sources via global deep-ocean currents (Ichino et al., 2015). Also, these and other tectonic trenches create nearby volcanic island arcs with undersea hydrothermal vents, which eject metal-rich plumes (see below), and mud volcanoes (Fryer, 1992) which at least in some areas of the world release high-density metals (Liu et al., 2012; Talas et al., 2015).
Notably, the Mariana Trench showed an overall a greater exposure (except for Ni and Cr; see below), perhaps from nearby Guam with its extensive military installations and/or because it is in the heavily populated Northern Hemisphere; regarding the Kermadec Trench, in the sparsely populated Southern Hemisphere, there are no inhabited islands near where fish were captured, and nearby New Zealand's North Island does not have major sources of metal pollutants. However, high accumulation of organic pollutants has recently been documented in both of these trenches by Jamieson et al. (2017); while the Mariana Trench had much higher levels of PCB (polychlorinated biphenyl) than the Kermadec, the pattern was reversed for PCBE (polybrominated diphenyl ether). Clearly pollutant sources need further study.
The single grenadier C. armatus from the Kermadec abyssal zone is also somewhat anomalous in having lower concentrations of most toxic elements than other deep-sea fish (compare abscissa scale of Fig. 2B to other panels of Fig. 2). For example, Hg content at 27 ppb was by far the lowest (Table 1; Fig. 2B). This species is a carnivore-scavenger like the snailfish, ranking at a slightly higher food-chain level with food items overlapping those of snailfish (Gerringer et al., 2017b). Thus the large differences in high density, toxic elemental contents between grenadier and snailfish may be due to habitat location and depth, rather than (or in addition to) diet. Notably, in another study of Hg in C. armatus caught in Monterey Bay, 2 individuals had Hg contents of 249 ppb and 630 ppb at 2000 and 3000 m, respectively (Moran, 2012). These values are similar to those of the flatfish and Kermadec snailfish (Table 1). Thus either the Kermadec grenadier has a relatively pristine abyssal habitat compared to Monterey Bay and the nearby Kermadec trench proper, or our results are misleading due having only 1 Kermadec grenadier specimen.
The hadal Kermadec trench snailfish exhibited much higher than expected levels of nickel and chromium (Fig. 4). We have confidence in this analysis because all the samples from the Kermadec and Mariana expeditions were performed with the same equipment, and the Kermadec grenadier (with very low toxic element contents) was caught on the same expedition as the Kermadec snailfish, which was only the species with concentrations of Ni and Cr at extreme levels. Moreover, all samples were digested and prepared at the same time with the same reagents. Analysis was performed at the same time in replicate. Any isobaric interferences would be consistent in all samples. A potential natural source of high density, toxic elements are the hydrothermal vents and volcanoes noted earlier. The Kermadec Trench, while it has no known hydrothermal activity, may have its own undersea volcanoes; notably, it is also close to the Southern Kermadec Arc volcanoes which have vents with high-density metals at much higher concentrations than found at the more well-known mid-ocean ridge vents (Massoth et al., 2003). Perhaps such metals collect in the trench (but not on the abyssal habitat of the grenadier) by the funnel mechanism (see above) or locally from trench volcanoes; however there is no evidence of or known mechanism for Ni and Cr being enriched relative to other metals. Possibly the Kermadec mud volcanoes are a source since—at least in one site, the Mediterranean—mud volcano sediments are highly enriched in Ni (but not Cr) relative to most other metals (Talas et al., 2015). We can offer no full explanation for the elevated concentrations of both of these metals in these fish.
4.2. Selenium to mercury ratios
One area of interest is the relative concentration of selenium to mercury in fish samples because of the relationship between mercury and selenoenzymes (Ralston and Raymond, 2018). It is well documented that selenium is an antagonist to Hg in a variety of species (Khan and Wang, 2009). The ratios in deep-sea sole (E. bathybius) and Dover sole (M. pacificus) (Table 2), which were sampled from depths of around 1000 meters, are particularly important because of the increasing expansion of commercial fishing operations into deeper waters. Although sample size is small (n = 3 for each species) and therefore relationships are hard to define, there is clearly a large molar excess of selenium in all of the fish sampled: the mean molar ratio of Se:Hg for these coastal fish combined (n = 6) was 18.4:1, well above the 1:1 standard recommended for human consumption (Perrault et al., 2013). The values range from 40:1 to 6.0:1 (Table 2; Fig. 6).
4.3. Mercury relative isotope analysis
Recently, groups such as Blum et al. (2013) have attempted to use isotopic analysis as a tracer of the source of Hg contamination in the environment. Those authors published results indicating a systematic decrease in Δ199Hg, Δ201Hg, δ202Hg values with increasing depth of occurrence, as well as a linear relationship between MIF and MDF (Bergquist and Blum, 2007). Our results do not show a linear relationship between delta values and depth (Fig. 6). Instead, we found a wide dispersion of relative isotopic ratios (statistical analysis was not applicable for the delta values because the fish were taken from different depths and sampling locations). The inability to find a relationship between depth and relative isotopic ratios are most likely a result of our low sample sizes and lack of consistency in sampling locations, and possible relatively low concentrations measured for some Hg isotopes needed in the calculation. Because our samples sizes were small enough and our results inconsistent, no source of mercury could be reliably designated. However, these data may be used as basis for comparison to future analyses of fish from other depths and locations.
5. Conclusion
Our results indicate that future studies will be worth conducting over a variety of ocean depths and habitats close and far from human pollutant sources. Identifying the source of mercury through isotopic profiling was unsuccessful because of the small sample size and wide distribution of relative isotopic ratios. Our lab will continue to analyze samples for high density, toxic elemental concentrations in an attempt to assess the significance of anthropogenic sources of high density, toxic element contamination. We have received a sediment core from Deep Lake in Washington, which will give insight into the natural levels of high density elements due to geothermal activity, as well as give information about the history of toxic high-density element pollution in the environment and the effect of human contribution.
Declarations
Author contribution statement
Connor Welty, Matthew Sousa, Frank Dunnivant: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Paul Yancey: Analyzed and interpreted the data; Contributed reagents, specimens, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Whitman College Perry Grants and the Student-Faculty Summer Research program, and grants to PHY (along with co-P.I.s) from the National Science Foundation, USA (NSF-OCE 1130494) and the Schmidt Ocean Institute (FK141109).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank the U.S. West Coast Groundfish Bottom Trawl Survey group and the captains and crews of the R/Vs Thompson and Falkor. For cruise organizing and assisting with collection and processing of specimens at sea, we are also grateful for the help of Jeffrey Drazen (chief scientist of Falkor cruise) and Mackenzie Gerringer (University of Hawai'i) for hadal trap design, Tim Shank (Woods Hole Oceanographic; chief scientist of Thompson cruise), and other members of the cruises' science parties. We are appreciative of the support and supplies provided by the National Science Foundation, USA; Schmidt Ocean Institute; and Whitman College Biology and Chemistry Departments.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- ATSDR (Agency for Toxic Substances and Disease Registry) Centers for Disease Control; Atlanta, GA: 2004. Toxicological Profile for Copper.https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=206&tid=37 [Google Scholar]
- Albare'de F., Telouk P., Blichert-Toft J., Boye M., Agranier A., Nelson B. Precise and accurate isotopic measurements using multiple-collector ICPMS. Geochim. Cosmochim. Acta. 2004;68:2725–2744. [Google Scholar]
- Bashir F.A., Shuhaimi-Othman M., Mazlan A.G. Evaluation of trace metal levels in tissues of two commercial fish species in Kapar and Mersing coastal waters, Peninsular Malaysia. J. Environ. Public Health. 2012;2012 doi: 10.1155/2012/352309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist B.A., Blum J.D. Mass-dependent and -independent fractionation of Hg isotopes by photo-reduction in aquatic systems. Science. 2007;318:417–420. doi: 10.1126/science.1148050. [DOI] [PubMed] [Google Scholar]
- Blum J.D., Erel Y. vol. 5. Elsevier; New York: 2003. Radiogenic isotopes in weathering and hydrology; pp. 365–392. (Treatise on Geochemistry). 12. [Google Scholar]
- Blum J.D., Popp B.N., Drazen J.C., Choy A.C., Johnson M.W. Mercury isotope evidence for methylation below the mixed layer in the central North Pacific Ocean. Nat. Geosci. 2013;6:879–884. [Google Scholar]
- Burger J., Gochfeld M., Burke S., Jeitner C.W., Jewett S., Snigaroff D., Snigaroff R., Stamm T., Harper S., Hoberg M., Chenelot H., Patrick R., Volz C.D., Weston J. Do scientists and fishermen collect the same size fish? Possible implications for exposure assessment. Environ. Res. 2006;101:34–41. doi: 10.1016/j.envres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Burger J., Gochfeld M., Jeitner C., Pittfield T., Donio M. Heavy metals in fish from the Aleutians: interspecific and locational differences. Environ. Res. 2014;131:119–130. doi: 10.1016/j.envres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.W., Magos L., Suzuki T., editors. Toxicology of Metals. CRC Press; Boca Raton, Florida: 1996. [Google Scholar]
- Chen C.Y., Stemberger R.S., Klaue B., Blum J.D., Pickhard P.C., Folt C.L. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol. Oceanogr. 2000;45:1525–1536. [Google Scholar]
- Chen J.B., Gaillardet J., Louvat P. Zinc isotopes in the Seine River waters, France: a probe of anthropogenic contamination. Environ. Sci. Technol. 2008;42:6494–6501. doi: 10.1021/es800725z. [DOI] [PubMed] [Google Scholar]
- Choy C.A., Popp B.N., Drazen J.C., Kaneko J.J. The influence of depth on mercury levels in pelagic fishes and their prey. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13865–13869. doi: 10.1073/pnas.0900711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrade N., Carignan J., Donard O.F.X. Isotope tracing of atmospheric mercury sources in an urban area of northeastern France. Environ. Sci. Technol. 2010;44:6062–6067. doi: 10.1021/es100674a. [DOI] [PubMed] [Google Scholar]
- Fitzgerald W.F., Lamborg C.H., Hammerschmidt C.R. Marine biogeochemical cycling of mercury. Chem. Rev. 2007;107:641–662. doi: 10.1021/cr050353m. [DOI] [PubMed] [Google Scholar]
- Fryer P. Mud volcanoes of the Marianas. Sci. Am. 1992;266:46–53. [Google Scholar]
- Gerringer M.E., Linley T.D., Jamieson A.J., Goetze E., Drazen J.C. Pseudoliparis swirei sp. nov.: a newly-discovered species of hadal snailfish (Scorpaeniformes: Liparidae) from the Mariana Trench. Zootaxa. 2017;4358:167–177. doi: 10.11646/zootaxa.4358.1.7. [DOI] [PubMed] [Google Scholar]
- Gerringer M.E., Popp B.N., Linley T.D., Jamieson A.J., Drazen J.C. Comparative feeding ecology of abyssal and hadal fishes through stomach content and amino acid isotope analysis. Deep Sea Res. I. 2017;121:110–120. [Google Scholar]
- Gworek B., Bemowska-Kałabun O., Marta Kijeńska M., Wrzosek-Jakubowska J. Mercury in marine and oceanic waters—a review. Water Air Soil Pollut. 2016;227:371. doi: 10.1007/s11270-016-3060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichino M.C., Clark M.R., Drazen J.C., Jamieson A., Jones D.O.B., Rowden A.A., Shank T.M., Yancey P.H., Ruhl H.A. The distribution of benthic biomass in hadal trenches: a modelling approach to investigate the effect of vertical and lateral organic matter transport to the seafloor. Deep Sea Res. I. 2015;100:21–33. [Google Scholar]
- Jamieson A.J., Malkocs T., Piertney S.B., Fujii T., Zhang Z. Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat. Ecol. Evol. 2017;1 doi: 10.1038/s41559-016-0051. 0051. [DOI] [PubMed] [Google Scholar]
- Khan M.A.K., Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009;28:1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- Larsson P., Holmqvist N., Stenroth P. Heavy metals and stable isotopes in a benthic omnivore in a trophic gradient of lakes. Environ. Sci. Technol. 2007;41:5973–5979. doi: 10.1021/es0704838. [DOI] [PubMed] [Google Scholar]
- Linley T., Gerringer M., Yancey P.H., Drazen J.C., Weinstock C., Jamieson A. Fishes of the hadal zone including new species, in situ observations and depth records of Liparidae. Deep Sea Res. I. 2016;114:99–110. [Google Scholar]
- Liu C.C., Maity J.P., Jean J.S., Selim Reza A.H.M., Li Z., Nath B. Geochemical characteristics of the mud volcano fluids in southwestern Taiwan and their possible linkage to elevated arsenic concentration in Chianan plain groundwater. Environ. Earth Sci. 2012;66:1513–1523. [Google Scholar]
- Massoth G.J., De Ronde C.E.J., Lupton J.E., Feely R.A., Baker E.T., Lebon G.T., Maenner S.M. Chemically rich and diverse submarine hydrothermal plumes of the southern Kermadec volcanic arc (New Zealand) Geol. Soc. Lond. Spec. Publ. 2003;219:119–139. [Google Scholar]
- Moran H. Whitman College; 2012. Analysis of Mercury in Deep-sea Grenadier.http://people.whitman.edu/∼yancey/Moran.Thesis.WhitmanCollege.2012.pdf (undergraduate thesis written as a degree requirement under the supervision of Paul H. Yancey) [Google Scholar]
- Perrault J.R., Miller D.L., Garner J., Wyneken J. Mercury and selenium concentrations in leatherback sea turtles (Dermochelys coriacea): population comparisons, implications for reproductive success, hazard quotients and directions for future research. Sci. Total Environ. 2013;463–464:61–71. doi: 10.1016/j.scitotenv.2013.05.067. [DOI] [PubMed] [Google Scholar]
- Perrot V., Epov V.N., Pastukhov M.V., Grebenshchikova V.I., Zouiten C., Sonke J.E., Husted S., Donard O.F., Amouroux D. Tracing sources and bioaccumulation of mercury in fish of Lake Baikal--Angara River using Hg isotopic composition. Environ. Sci. Technol. 2010;44:8030–8037. doi: 10.1021/es101898e. [DOI] [PubMed] [Google Scholar]
- Ralston N.V.C., Raymond L.J. Mercury's neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta. 2018;1862:2405–2416. doi: 10.1016/j.bbagen.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Scudder B.C., Chasar L.C., Wentz D.A., Bauch N.J., Brigham M.E., Moran P.W., Krabbenhoft D.P. U.S. Geological Survey Scientific Investigations; 2009. Mercury in Fish, Bed Sediment, and Water from Streams across the United States, 1998-2005. Report 2009-5109. [Google Scholar]
- Slowey A.J., Brown G.E. Transformations of mercury, iron, and sulfur during the reductive dissolution of iron oxyhydroxide by sulfide. Geochim. Cosmochim. Acta. 2007;71:877–894. [Google Scholar]
- Sun R., Sonke J.E., Heimbürger L.-E., Belkin H.E., Liu G., Shome D., Cukrowska E., Liousse C., Pokrovsky O.S., Streets D.G. Mercury stable isotope signatures of world coal deposits and historical coal combustion emissions. Environ. Sci. Technol. 2014;48:7660–7668. doi: 10.1021/es501208a. [DOI] [PubMed] [Google Scholar]
- Stern B.R. Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. Toxicol. Environ. Health A. 2010;73:114–127. doi: 10.1080/15287390903337100. [DOI] [PubMed] [Google Scholar]
- Talas E., Duman M., Küçüksezgin F., Brennan M.L., Raineault N.A. Sedimentology and geochemistry of mud volcanoes in the Anaximander Mountain Region from the Eastern Mediterranean Sea. Mar. Poll. Bull. 2015;95:63–67. doi: 10.1016/j.marpolbul.2015.04.042. [DOI] [PubMed] [Google Scholar]
- Tchounwou P.B., Yedjou C.G., Pattola A.K., Sutton D.J. Heavy metal toxicity and the environment. In: Luch A., editor. Molecular, Clinical and Environmental Toxicology. vol. 101. Springer; Basel: 2012. (Experientia Supplementum). [Google Scholar]
- Wiederhold J.G. Metal stable isotope signatures as tracers in environmental geochemistry. Environ. Sci. Technol. 2015;49:2606–2624. doi: 10.1021/es504683e. [DOI] [PubMed] [Google Scholar]
- Welz B., Šucmanová M. L-cysteine as a reducing and releasing agent for the determination of antimony and arsenic using flow injection hydride generation atomic absorption spectrometry—Part 1. Optimization of the analytical parameters. Analyst. 1993;11:1417–1423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.