Abstract
Total pancreatectomy and islet autotransplantation (TPIAT) is an effective treatment for selected patients with chronic pancreatitis. The portal circulation is the standard infusion site for islet transplant, but marked elevation of portal pressures may prevent complete islet infusion. Herein we report a novel technique of combined site islet autotransplantation using an omental pouch. This technique may be useful when technical limitations prevent complete intraportal transplantation. In four TPIAT recipients with intraoperative issues precluding the complete intraportal infusion of islets, an omental pouch was created to contain the remaining islet mass. Patients were monitored for complications, and islet graft function was assessed using mixed meal tolerance testing and compared with matched controls who received only intraportally transplanted islets. All patients had decreasing insulin requirements as their recovery progressed. At 3 months follow-up there were no significant differences in glycemic control or graft function for the combined site recipients compared with their matched controls who only received an intraportal islet infusion. The omentum has potentially desirable qualities such as accessibility, capacity, and systemic/portal vascularity comparable to the native pancreas. The omental pouch technique may represent a safe and effective alternate site for islet autotransplantation. Further study is needed to confirm these findings.
Keywords: omental pouch, total pancreatectomy islet autotransplantation, chronic pancreatitis, islet autotransplantation, extrahepatic islet autotransplantation
Introduction
Total pancreatectomy and islet autotransplantation (TPIAT), first performed in 1977, is an effective treatment for relief of pain from severe chronic pancreatitis (CP) while reducing the likelihood of brittle diabetes associated with pancreatectomy alone1,2. In current practice, the portal venous system is the primary site for infusion of isolated islets due to its ease of use for islet infusion and demonstrated efficacy for restoring beta cell function. However, the intraportal infusion site carries several important limitations including the risk for portal vein thrombosis and bleeding that can occur related to elevated portal pressures, and the early loss of islet mass resulting from the instant blood-mediated inflammatory response (IBMIR) triggered by the islet infusion3–5. In cases where portal pressures remain elevated during infusion, alternative sites for transplantation of the remaining islets are used, since total transplanted islet mass is a critical determinant of future insulin independence6,7. Many alternate sites have been explored, including but not limited to the omentum, peritoneum, gastric submucosa, and renal subcapsule; however, these sites are associated with limitations of capacity, complications, or questionable functional outcome8–18. The omentum is an attractive alternative site because it is easily accessible intraoperatively, lacks volume restrictions, and is highly vascularized with portal venous drainage simulating the native pancreatic endocrine pathway8,9.
Baidal et al. recently reported a patient achieving insulin independence during 1 year of follow-up after an islet allotransplantation into the omentum in a type 1 diabetic recipient19, providing evidence the omentum can be used as an alternative to intraportal islet transplantation. There are important contrasts between islet allo- and autotransplantation. Islet allografts are smaller volume, purified islet preparations administered concomitantly with immunosuppression. In contrast, autografts are typically unpurified cell preparations that contain a larger amount of exocrine tissue, with different magnitudes of innate inflammatory response and apoptosis that may impact the early engraftment of the islet mass. Autograft patients do not require immunosuppression.
Herein we report the early outcomes of the first series of patients who received a portion of their islet autotransplantation into the omentum after intraoperative features precluded complete intraportal islet infusion.
Case Report
Case 1
A 35-year-old woman presented after multiple episodes of relapsing acute pancreatitis, which progressed to CP despite multiple endoscopic interventions over the course of 7 years. Given that her CP was complicated by persistent debilitating pain and continued weight loss, the patient was evaluated by our multidisciplinary team and deemed an appropriate candidate for TPIAT. She was not diabetic at the time of her procedure. Her islet isolation produced 6,822 IEQ/kg in a 17.5 mL tissue volume. During the islet infusion, the patient’s portal pressure elevation exceeded our safety threshold of 25 cm H2O above baseline; therefore, it was elected to transplant the remainder (25%) of her islet preparation into the greater omentum near the transverse colon, employing the omental pouch technique described below.
Case 2
A 63-year-old woman was diagnosed with CP attributed to sphincter of Oddi dysfunction 4 years prior to TPIAT. She had failed maximal endoscopic therapy and eventually required feeding tube placement for severe pain with oral intake and consequent malnutrition. The patient was deemed to be a candidate for TPIAT after careful review. The patient was not diabetic prior to surgery. The islet yield after processing was 2,750 IEQ/kg in a tissue volume of 15 mL. While infusing islets into the portal venous system, there was an increase in portal pressure greater than 25 cm H2O above baseline, and the remainder (36%) of her islets were placed in an omental pouch fashioned from the greater omentum near the transverse colon.
Case 3
The patient is a 49-year-old man with CP secondary to alcohol consumption with pain for 2 years prior to his operation. The patient failed endoscopic intervention and required a jejunal feeding tube secondary to severe pain with oral intake. He had no insulin requirements at the time of operation but was pre-diabetic by glycosylated hemoglobin of 5.9% and a fasting glucose of 101 mg/dL. He reported alcohol abstinence, a requirement at our institution, leading up to his procedure. The patient’s islet yield was 3,786 IEQ/kg in a tissue volume of 24 mL following pancreas processing. During intraportal islet infusion, the catheter was inadvertently displaced and the remainder (12%) of his islet yield was transplanted into an omental pouch.
Case 4
The patient is a 26-year-old woman with relapsing acute pancreatitis secondary to abdominal trauma suffered during a motor vehicle accident 6 years prior to her procedure. Despite attempts at endoscopic management, the patient’s pain increased and she eventually required a gastro-jejunal feeding tube secondary to constant pain and vomiting associated with oral intake. Following a careful review by our multidisciplinary team, she was deemed an appropriate candidate for TPIAT. The patient was not diabetic at the time of her procedure. Her islet yield was 6,778 IEQ/kg in a 40 mL tissue volume. During the islet infusion, the catheter was displaced. At the time of displacement, the patient’s portal pressure was 25 cm H2O above baseline, and the decision was made not to replace the catheter and the remainder (36%) of her islets were placed in an omental pouch.
Materials and Methods
Surgical Technique
Total pancreatectomy and islet isolation were performed as previously described20,21. At our institution, islets are infused following gastrointestinal reconstruction. Heparin (70 units/kg) is administered, and the portal venous system is accessed by cannulation of the splenic vein, middle colic vein, or superior mesenteric vein. The islet preparation is infused via gravity into the intrahepatic portal venous circulation. A manometer is used to monitor portal pressures prior to infusion, halfway through infusion, and at the end of infusion. If the change in portal pressure during islet infusion rises >25 cm H2O above baseline, infusion is paused and pressure is reevaluated after an additional 15 minutes. If portal pressures remain elevated above the 25 cm H2O threshold, the portal infusion is discontinued. In the aforementioned cases, the remaining islets were reserved for transplantation into an omental pouch.
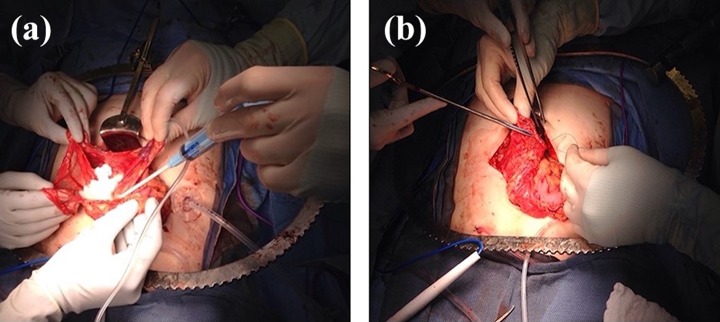
In preparation for omental transplantation, the islets are allowed to settle from their solution to the bottom of the infusion bag before being drawn into a 60 cc syringe. A “bowl” of omentum is created by lifting up edges of the omentum from the transverse colon (Fig. 1a). The islets are dripped into the “bowl” and Evicel (Johnson & Johnson Wound Management, Somerville, NJ), a fibrin sealant, is applied over the islets, helping to affix them to the omentum to facilitate engraftment. The omental pouch is closed using a running suture (Fig. 1b) and surgical clips are placed on the borders of the pouch to aid in later identification of the area of transplant.
Figure 1.
Omental pouch creation. (a) Pouch is created by lifting up edges of omentum. The concentrated islet preparation is dripped on to the omentum and affixed using a fibrin sealant hemostatic agent. (b) After islets have been affixed with the hemostatic agent, the pouch is sealed with a running 3-0 Vicryl suture.
Mixed Meal Tolerance Testing
Post-operatively, all patients are managed with insulin to promote strict euglycemia for approximately 3 months and prevent undue stress and resultant apoptosis of the islets during engraftment22,23. At 3 months post-operatively, patients undergo a mixed meal tolerance test (MMTT) to assess graft function. After an overnight fast, patients drink Boost High Protein (HP) (Nestle Health Science, Bridgewater, NJ, USA) 6 mL/kg body weight (maximum 360 mL). Serum glucose and C-peptide levels are obtained at 0, 60, and 120 minutes to measure the response to Boost HP consumption. Hemoglobin A1c (HbA1c) is measured as a marker of average glycemic control, and insulin dose is assessed by patient report or patient logbook.
Control Patient Selection
A control population was selected from the TPIAT database maintained by the University of Minnesota. Control patients had undergone TPIAT with all islets infused via the intraportal route. Two control patients were matched with each of the four omental pouch cases described above based on sex, age, body mass index (BMI), and IEQ/kg (Table 1). Patients were excluded from consideration as potential matched controls if they had diabetes prior to treatment, as none of the patients receiving the omental pouch technique had diabetes or insulin requirements prior to operation. Patients were also excluded from consideration if they had incomplete follow-up data.
Table 1.
Characteristics of Cases and Controls.
| Patient 1 | Controls | Patient 2 | Controls | Patient 3 | Controls | Patient 4 | Controls | |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | F/F | Female | F/F | Male | M/M | Female | F/F |
| TPIAT year | 2017 | 2010/2011 | 2017 | 2011/2011 | 2017 | 2012/2014 | 2017 | 2011/2011 |
| Age (years) | 35 | 38 | 63 | 58 | 49 | 54 | 26 | 22 |
| BMI (kg/m2) | 19.0 | 21.8 | 20.6 | 22.1 | 27.7 | 26.0 | 18.4 | 20.1 |
| Islet yield (IEQ*/kg) | 6,822 | 6,375 | 2,750 | 2,785 | 3,786 | 3,731 | 6,778 | 6,939 |
| Omental islets (%) | 25 | 0 | 36 | 0 | 12 | 0 | 36 | 0 |
Mean values displayed for control patients unless both values are displayed.
TPIAT = Total pancreatectomy islet autotransplantation, BMI = Body mass index, IEQ = Islet equivalent.
* Islet equivalent defined as a pancreatic islet with a diameter of 150 µm.
Statistical Analysis
Omental pouch patients were compared with their matched intraportal controls using a two-tailed Student’s t-test to evaluate for differences in glycemic control and islet graft function. Glycemic control was measured using fasting glucose and 120 minute glucose from MMTT, HbA1c, and daily basal insulin. Peak C-peptide from MMTT was used to assess islet graft function. Statistical significance was set at 0.05, and the Bonferroni correction method was applied to account for multiple statistical comparisons made between groups. All analyses were performed using R Version 3.2.1 (Vienna, Austria) statistical software.
Results
Hospital Course and Early Follow-up
None of the four omental pouch recipients experienced intraoperative complications. Post-operatively the first patient’s islet preservation solution grew Streptococcus mitis, and per our standard protocol, she completed a 7 day course of antibiotics. She was discharged to a local extended stay residence on post-operative day (POD) 13 requiring 11 units of basal insulin daily. The patient was re-hospitalized on POD 52 for delayed gastric emptying and Clostridium difficile infection. The second patient required 1 unit of packed red blood cells post-operatively for acute blood loss anemia but had an otherwise uncomplicated recovery. She was discharged home on POD 10 requiring 36 units of basal insulin daily. For the third patient, an emergent exploratory laparotomy was required on POD 3 in the setting of increasing abdominal pain and distension. The exploration was negative for any acute processes requiring correction and the omental pouch was successfully identified and found to be intact. After his negative exploratory laparotomy, it was discovered the patient had been consuming alcohol leading up to his operation and was undergoing acute alcohol withdrawal. He subsequently developed a superficial skin infection and pneumonia. The patient was discharged home on POD 40 requiring 48 units of daily basal insulin. The fourth patient’s islet preservation solution grew Enterobacter and she completed a 5-day course of antibiotics. Later during her hospitalization, she developed hospital-acquired pneumonia. She was minimally symptomatic from the pneumonia and successfully completed a course of antibiotics. The patient was discharged home on POD 12 requiring 44 units of daily basal insulin for glycemic control. All patients were on enteral tube feeds for nutrition at the time of discharge which were later discontinued following surgical recovery.
Graft Function
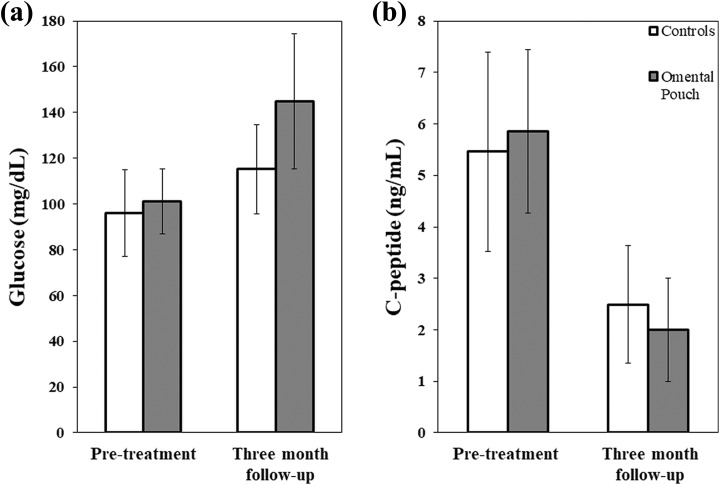
All patients maintained close clinical follow-up after their discharge from the hospital and progressively decreased their daily basal insulin requirements. At 3 months post-operatively, patient 1 required 6 units of basal insulin, patient 2 required 7 units, patient 3 required 13 units, and patient 4 required 5 units. All patients saw an increase in their HbA1c from their pre-operative levels with values of 5.5, 5.9, 6.1, and 5.4, respectively. MMTT was conducted at 3 months after surgery and compared with their pre-operative baseline (Fig. 2). As expected in the early post-operative course, glucose levels rose and C-peptide levels fell in comparison to pre-operative measurements, but all omental pouch recipients undergoing post-operative graft function assessments had partial islet graft function based on C-peptide levels. At 3 months post-operatively, the cases with a partial omental pouch infusion had similar fasting glucose, 120 minute glucose and peak C-peptide measurements to the matched control group (Table 2). Additional diabetes outcomes HbA1c and daily basal insulin use demonstrated no significant differences between omental pouch and control patients.
Figure 2.
Comparison of pre-TPIAT and 3-month follow-up glycemic function in omental pouch recipients and their matched controls. Histogram bars represent mean pooled values for all patients in a respective group. Error bars show a single standard deviation. (a) 120 minute glucose results. No significant differences between groups. (b) Peak C-peptide, represented by maximum C-peptide value obtained during MMTT. No significant differences between groups.
Table 2.
Comparison of graft function between cases and controls at 3 months post-operatively.
| Omental pouch | Controls | ||
|---|---|---|---|
| n = 4 | n = 8 | P-value | |
| MMTT Peak C-peptide (ng/mL) | 2.0 (1.0) | 2.5 (1.1) | 1 |
| Fasting glucose (mg/dL) | 111 (20.5) | 84 (28.4) | .61 |
| MMTT 120 minute glucose (mg/dL) | 145 (29.5) | 115 (19.5) | .31 |
| Hemoglobin A1c (%) | 5.8 (0.33) | 5.9 (0.33) | 1 |
| Basal insulin dose (U/day) | 7.8 (3.6) | 7.4 (4.1) | 1 |
All values are continuous, represented with mean (SD).
MMTT = Mixed meal tolerance test.
Discussion
The patients receiving islet autotransplantation utilizing a combined intraportal and omental pouch technique had similar early post-operative outcomes compared with patients receiving their entire islet mass intraportally. Early graft function and glycemic control suggest combined site outcomes are comparable to the complete intraportal recipients. Based on these early results, transplanting a portion of the islet mass via the omental pouch technique represents a safe and practical treatment option for patients unable to tolerate a complete intraportal infusion of the islet preparation.
The portal venous system is the most common site for islet autotransplantation; however, it has several shortcomings. As described earlier, elevated portal pressures from intraportal islet infusion can produce life-threatening complications such as bleeding and portal vein thrombosis. Elevated portal pressures have been associated with transplanting large-volume islet preparations intraportally4. Islet preparation volume reduction is possible through density gradient purification. However, this process significantly reduces islet mass24 thereby lessening the chance for future insulin independence, and thus purification is generally avoided at our institution unless the tissue volume exceeds 0.25 mL/kg body weight. Insulin independence has been shown to provide a significantly higher physical quality of life in patients undergoing TPIAT25, thus preserving the islet mass is of great importance.
Rates of insulin independence decrease over time after undergoing TPIAT, due to loss of transplanted islet mass. One suspected early contributor to islet loss is the IBMIR, a nonspecific inflammatory reaction involving coagulation, complement activation, and inflammatory cell infiltration. IBMIR has been well characterized in allograft islet transplants, negatively affecting islet survival, and more recently has been demonstrated in an islet autotransplantation in vitro model5. Direct blood contact is required for IBMIR, making any intravascular transplant site, such as the portal venous system, a risk factor for islet loss and thrombotic complications26. Given the complications and significant islet loss associated with IBMIR, extrahepatic sites for islet autotransplantation have been investigated as potential alternatives.
While numerous sites have been investigated (Table 3), the omentum offers distinct advantages including capacity, accessibility, and physiologic environment. The omentum lacks any volume restrictions11, allowing consistent complete islet mass transplantation. The omentum is easily accessible during TPIAT, and can easily be found later if needed for subsequent evaluation or biopsy, as evidenced by our experiences with our omental pouch patient that required a re-operation. Furthermore, the omentum provides portal venous drainage and good arterial supply for nutrient delivery, mimicking native islet physiology.
Table 3.
Other Described Extrahepatic Sites Used for Islet Autotransplantation.
| Extrahepatic site | Advantages | Disadvantages | Level of investigation | Reference |
|---|---|---|---|---|
| Omentum | Large transplant capacity Portal venous drainage Easily accessible |
Large islet requirement Delayed engraftment |
Nonhuman primate | Yasunami et al.8
Wahoff et al.9 Berman et al.10 Ao et al.11 |
| Peritoneum | Large transplant capacity Easily accessible |
Large islet requirement Difficult reidentification |
Canine | Wahoff et al.9
Fritschy et al.12 |
| Gastric submucosa | Rich vascularization Easy glycemic monitoring |
Poorer graft function* Gastrointestinal injury risk |
Canine | Yin et al.13 |
| Renal subcapsule | Specific location |
Large islet requirement Diminished glycemic control* |
Nonhuman primate | Rajab et al.14
Kaufmann et al.15 |
| Intramuscular | Minimally invasive Low injury risk |
Poor early vascularization Diminished glycemic control* |
Porcine | Sterkers et al.16
Sakata et al.17 |
| Bone marrow | Easily accessible Rich vascularization |
Few clinical studies | Human | Maffi et al.18 |
| Subcutaneous | Minimally invasive | Poor early vascularization Low oxygen tension |
Rat | Sakata et al.17 |
* Compared with intraportal islet autotransplantation, the primary transplant site in current practice.
Although the omentum has substantial benefits, previous research has suggested that it requires a larger islet mass for successful graft function and has a longer period before function is observed10. The peritoneum and gastric submucosa offer other easily accessible options during TPIAT, and have been historically used at our institution if the entire islet preparation volume cannot be placed intraportally. The peritoneum, like the omentum, lacks volume restrictions; however, evidence for post-operative glycemic control is inconsistent. In nonhuman studies, it has been suggested insufficient parasympathetic reinnervation to the islet graft leads to abnormal glucose tolerance12, but other work suggests glycemic control can be attained with a sufficiently large transplanted islet mass9. In addition, subsequent identification of the islet graft is difficult. The gastric submucosa has portal venous drainage and vascular density suggesting a potentially successful islet transplant site, but islet autotransplantation in canines showed poorer graft function when compared with the intraportal site13.
Our report describes a simple technique used for omental pouch islet autotransplantation which maximizes contact surface area for the transplanted islets, a desirable aspect noted in other studies27,28. Previous research has discussed preventing islet leakage by using sutures to close the omental pouch, but suturing should be minimized to lessen the disruption of omental revascularization28. Islet survival is heavily influenced by the microenvironment, and three-dimensional scaffoldings have been shown to recreate the islet microenvironment better than free-floating islets by preventing clumping and promoting vascularization29. We created a fibrin scaffold produced by applying a hemostatic gel agent on top of the transplanted islets. Using a technique with similar methodology, Baidal et al.19 published results from a patient receiving islet omental allotransplantation for type 1 diabetes. The authors layered the islets on to the omentum and secured the islets with a fibrin scaffold derived from plasma obtained from the transplant recipient. Shortly after the procedure, their patient achieved insulin independence. At 12 months, the patient maintained insulin independence without hypoglycemic events.
The complications that were experienced by these patients were likely not attributable to the omental pouch technique. Delayed gastric emptying and Clostridium difficile infection are associated with TPIAT and antibiotic use, respectively, and the new technique is unlikely to have produced these complications30,31. Patient 3 had several complications during his hospital course. Acute alcohol withdrawal, despite universal counseling for pre-operative alcohol abstinence, was a significant contributor to his prolonged hospitalization. In addition the pneumonia and superficial skin infection he developed are unfortunately common complications associated with major surgery, and it is unlikely the omental pouch technique placed him at an increased risk for these complications. Notably, two of the omental pouch recipients had bacterial growth in their islet preservation solution and received antibiotic treatment. Research has shown positive cultures are common in TPIAT recipients regardless of transplant site, occurring in 61% of patients32. While it is a common event, less than 5% of patients with a positive culture have an infectious complication from the same organism grown from the preservation solution, suggesting it is unlikely to cause an infection in TPIAT patients.
Our study possesses several limitations that must be recognized. First, we have a small sample size with four patients utilizing the omental pouch transplant site. While similar early post-operative graft function was observed between groups, this could be an artifact of a limited sample. Second, we have a short post-operative follow-up period available. At the time of publication, our patients had only reached their 3-month follow-up when graft function is first assessed after TPIAT. Recovery from TPIAT and islet engraftment can be a lengthy process, and 3 months is an early time point. It is unknown whether long-term islet transplant function will remain similar for the two groups. Third, metabolic testing using MMTT and HbA1c assesses the overall graft function and is unable to specifically determine the viability of islets transplanted to the omental pouch. Therefore, it is possible the metabolic outcomes observed in omental pouch recipients are driven by the islets transplanted intraportally.
Conclusions
The omental pouch technique appears to be a safe and functional alternate site for islet autotransplantation which can be useful when complete intraportal infusion is precluded. The patients in which this technique was performed experienced similar early graft function and glycemic control compared with patients receiving their entire islet mass intraportally. Further studies involving a larger sample of omental pouch recipients with longer follow-up are necessary to more completely assess the efficacy of this technique; however, based on our limited experience, the omental pouch technique is a reasonable alternative when a completely intraportal islet autotransplantation is contraindicated.
Footnotes
Ethical Approval: This study was approved by the Institutional Review Board of the University of Minnesota.
Statement of Human and Animal Rights: The Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Institutional Review Board of the University of Minnesota).
Statement of Informed Consent: Written informed consent was obtained from all patients.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Mariya Skube is supported by NIH/NIDDK T32DK108733.
References
- 1. Sutherland DER, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin N Am. 1978;58(2):365–382. [DOI] [PubMed] [Google Scholar]
- 2. Chinnakotla S, Beilman GJ, Dunn TB, Bellin MD, Freeman ML, Radosevich DM, Arain M, Amateau SK, Mallery JS, Schwarzenberg SJ, Clavel A, Wilhelm J, Robertson RP, Berry L, Cook M, Hering BJ, Sutherland DE, Pruett TL. Factors predicting outcomes after a total pancreatectomy and islet autotransplantation lessons learned from over 500 cases. Ann Surg. 2015;262(4):610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilhelm JJ, Bellin MD, Dunn TB, Balamurugan AN, Pruett TL, Radosevich DM, Chinnakotla S, Schwarzenberg SJ, Freeman ML, Hering BJ, Sutherland DE, Beilman GJ. Proposed thresholds for pancreatic tissue volume for safe intraportal islet autotransplantation after total pancreatectomy. Am J Transplant. 2013;13(12):3183–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsumoto S, Takita M, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle JA, Tamura Y, Rahman AM, Onaca N, Naziruddin B, Levy MF. Impact of tissue volume and purification on clinical autologous islet transplantation for the treatment of chronic pancreatitis. Cell Transplant. 2012;21(4):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. 2014;14(2):428–437. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad SA, Lowy AM, Wray CJ, D’Alessio D, Choe KA, James LE, Gelrud A, Matthews JB, Rilo HL. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201(5):680–687. [DOI] [PubMed] [Google Scholar]
- 7. Bellin MD, Balamurugan AN, Pruett TL, Sutherland DE. No islets left behind: islet autotransplantation for surgery-induced diabetes. Curr Diab Rep. 2012;12(5):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yasunami Y, Lacy PE, Finke EH. A new site for islet transplantation-a peritoneal-omental pouch. Transplantation. 1983;36(2):182–183. [DOI] [PubMed] [Google Scholar]
- 9. Wahoff DC, Sutherland DE, Hower CD, Lloveras JK, Gores PF. Free intraperitoneal islet autografts in pancreatectomized dogs - impact of islet purity and posttransplantation exogenous insulin. Surgery. 1994;116(4):742–748. [PubMed] [Google Scholar]
- 10. Berman DM, O’Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ao Z, Matayoshi K, Lakey JR, Rajotte RV, Warnock GL. Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation. 1993;56(3):524–529. [DOI] [PubMed] [Google Scholar]
- 12. Fritschy WM, van Straaten JF, de Vos P, Strubbe JH, Wolters GH, van Schilfgaarde R. The efficacy of intraperitoneal pancreatic islet isografts in the reversal of diabetes in rats. Transplantation. 1991;52(5):777–783. [DOI] [PubMed] [Google Scholar]
- 13. Yin ZZ, Wang SS, Li Q, Huang Y, Chen L, Chen G, Liu R, Wang XM. Gastric submucosa is inferior to the liver as transplant site for autologous islet transplantation in pancreatectomized diabetic beagles. J Huazhong Univ Sci Technol. 2016;36(4):529–533. [DOI] [PubMed] [Google Scholar]
- 14. Rajab A, Buss J, Diakoff E, Hadley GA, Osei K, Ferguson RM. Comparison of the portal vein and kidney subcapsule as sites for primate islet autotransplantation. Transplantation. 2008;17(9):1015–1023. [DOI] [PubMed] [Google Scholar]
- 15. Kaufman DB, Morel P, Field MJ, Munn SR, Sutherland DE. Purified canine islet autografts. functional outcome as influenced by islet number and implantation site. Transplantation. 1990;50(3):385–391. [PubMed] [Google Scholar]
- 16. Sterkers A, Hubert T, Gmyr V, Torres F, Baud G, Delalleau N, Vantyghem MC, Kerr-Conte J, Caiazzo R, Pattou F. Islet survival and function following intramuscular autotransplantation in the minipig. Am J Transplant. 2013;13(4):891–898. [DOI] [PubMed] [Google Scholar]
- 17. Sakata N, Aoki T, Yoshimatsu G, Tsuchiya H, Hata T, Katayose Y, Egawa S, Unno M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab Res Rev. 2014;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 18. Maffi P, Balzano G, Ponzoni M, Nano R, Sordi V, Melzi R, Mercalli A, Scavini M, Esposito A, Peccatori J, Cantarelli E, Messina C, Bernardi M, Del Maschio A, Staudacher C, Doglioni C, Ciceri F, Secchi A, Piemonti L. Autologous pancreatic islet transplantation in human bone marrow. Diabetes. 2013;62(10):3523–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, Ciancio G, Linetsky E, Pileggi A, Alejandro R. Bioengineering of an intraabdominal endocrine pancreas. N Engl J Med. 2017;376(19):1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chinnakotla S, Bellin MD, Schwarzenberg SJ, Radosevich DM, Cook M, Dunn TB, Beilman GJ, Freeman ML, Balamurugan AN, Wilhelm J, Bland B, Jimenez-Vega JM, Hering BJ, Vickers SM, Pruett TL, Sutherland DE. Total pancreatectomy and islet auto-transplantation in children for chronic pancreatitis. indication, surgical techniques, post operative management, and long-term outcomes. Ann Surg. 2014;260(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, Soltani SM, Radosevich DM, Yuasa T, Tiwari M, Papas KK, McCarthy R, Sutherland DE, Hering BJ. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93(7):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juang JH, Bonner-Weir S, Wu YJ, Weir GC. Beneficial influence of glycemic control upon the growth and function of transplanted islets. Diabetes. 1994;43(11):1334–1339. [DOI] [PubMed] [Google Scholar]
- 23. Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. dynamic changes in structure and function. Diabetes. 1996;45(9):1161–1167. [DOI] [PubMed] [Google Scholar]
- 24. Morrison CP, Wemyss-Holden SA, Dennison AR, Maddern GJ. Islet yield remains a problem in islet autotransplantation. Arch Surg. 2002;137(1):80–83. [DOI] [PubMed] [Google Scholar]
- 25. Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B, Balamurugan AN, Freeman ML, Pruett TL. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK. The choice of anatomical site for islet transplantation. Cell Transplantation. 2008;17(9):1005–1014. [PubMed] [Google Scholar]
- 27. Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C, Pileggi A. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes. 2016;65(5):1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hefty TR, Kuhr CS, Chong KT, Guinee DG, Wang W, Reems JA, Greenbaum CJ. Omental roll-up: a technique for islet engraftment in a large animal model. J Surg Res. 2010;161(1):134–138. [DOI] [PubMed] [Google Scholar]
- 29. Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JR, Hart ME, Hayek A. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes. 2002;51(12):3435–3439. [DOI] [PubMed] [Google Scholar]
- 30. John GK, Singh VK, Pasricha PJ, Sinha A, Afghani E, Warren D, Sun Z, Desai N, Walsh C, Kalyani RR, Hall E, Hirose K, Makary MA, Stein EM. Delayed gastric emptying (DGE) following total pancreatectomy with islet auto transplantation in patients with chronic pancreatitis. J Gastrointest Surg. 2015;19(7):1256–1261. [DOI] [PubMed] [Google Scholar]
- 31. Slimings C, Riley TV. Antibiotics and hospital acquired clostridium difficile infection: updated of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(4):881–891. [DOI] [PubMed] [Google Scholar]
- 32. Colling KP, Blondet JJ, Balamurugan AN, Wilhelm JJ, Dunn T, Pruett TL, Sutherland DE, Chinnakotla S, Bellin M, Beilman GJ. Positive sterility cultures of transplant solutions during pancreatic islet autotransplantation are associated infrequently with clinical infection. Surg Infect. 2015;16(2):115–123. [DOI] [PubMed] [Google Scholar]