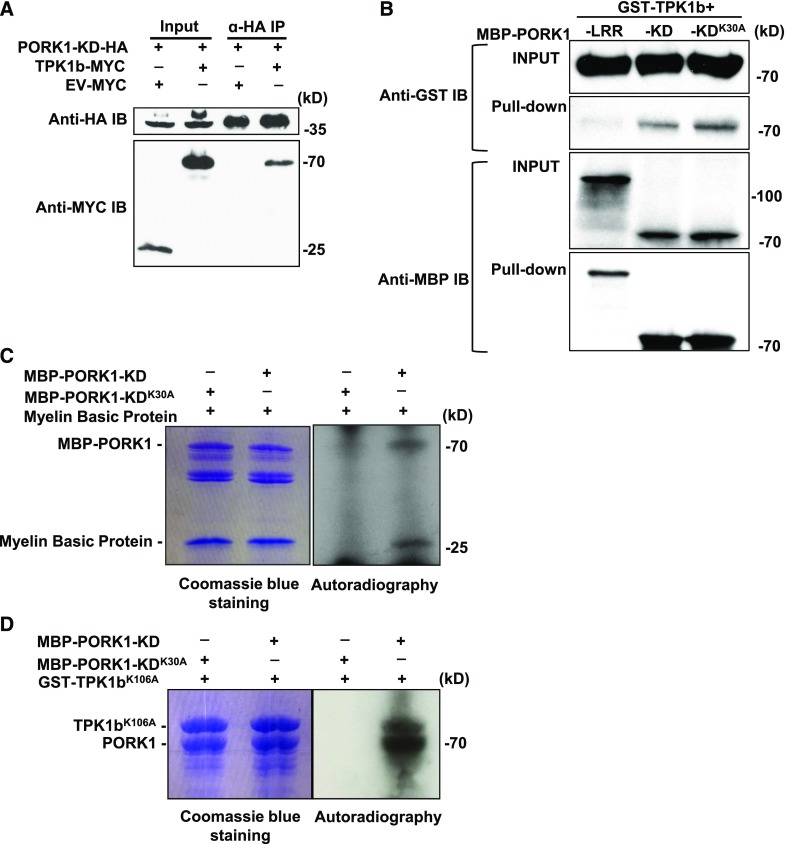
Figure 1.
PORK1 Interaction and Phosphorylation with TPK1b.
(A) TPK1b interacts with PORK1 kinase domain (KD). TPK1b-MYC and the kinase domain of PORK1 (PORK1-KD-HA) were coexpressed in N. benthamiana through Agrobacterium tumefaciens-mediated transient expression. PORK1-KD-HA was immunoprecipitated (IP) with anti-HA antibody coupled agarose beads along with TPK1b-MYC. The bound proteins were detected by immunoblot with the indicated antibodies.
(B) Direct interaction between PORK1 and TPK1b in in vitro binding assay. Equal amounts of recombinant proteins were mixed as shown in the input. After washing, GST-TPK1b was bound with MBP-PORK1-KD and MBP-PORK1-KDK30A, but not with the MBP-PORK1-LRR, which is used as a negative control.
(C) PORK1 is a functional kinase with autophosphorylation and transphosphorylation activities.
(D) PORK1 phosphorylates TPK1b in in vitro kinase assay.
In (C) and (D), MBP-PORK1-KD, MBP-PORK1-KDK30A, or GST-TPK1bK106A were expressed and purified from E. coli using amylose resin columns. MBP-PORK1-KD or MBP-PORK1-KDK30A was incubated with Myelin Basic Protein (C) or TPK1bK106A (D) in a kinase buffer containing [γ-32P]ATP. Phosphorylation was detected by autoradiography. Coomassie blue staining shows equal loading of protein samples. EV, empty vector. The experiments were repeated at least two times with similar results.