Figure 9.
The C Terminus of ERD2 Controls Efficient ER Export and Is Essential for Its Biological Activity.
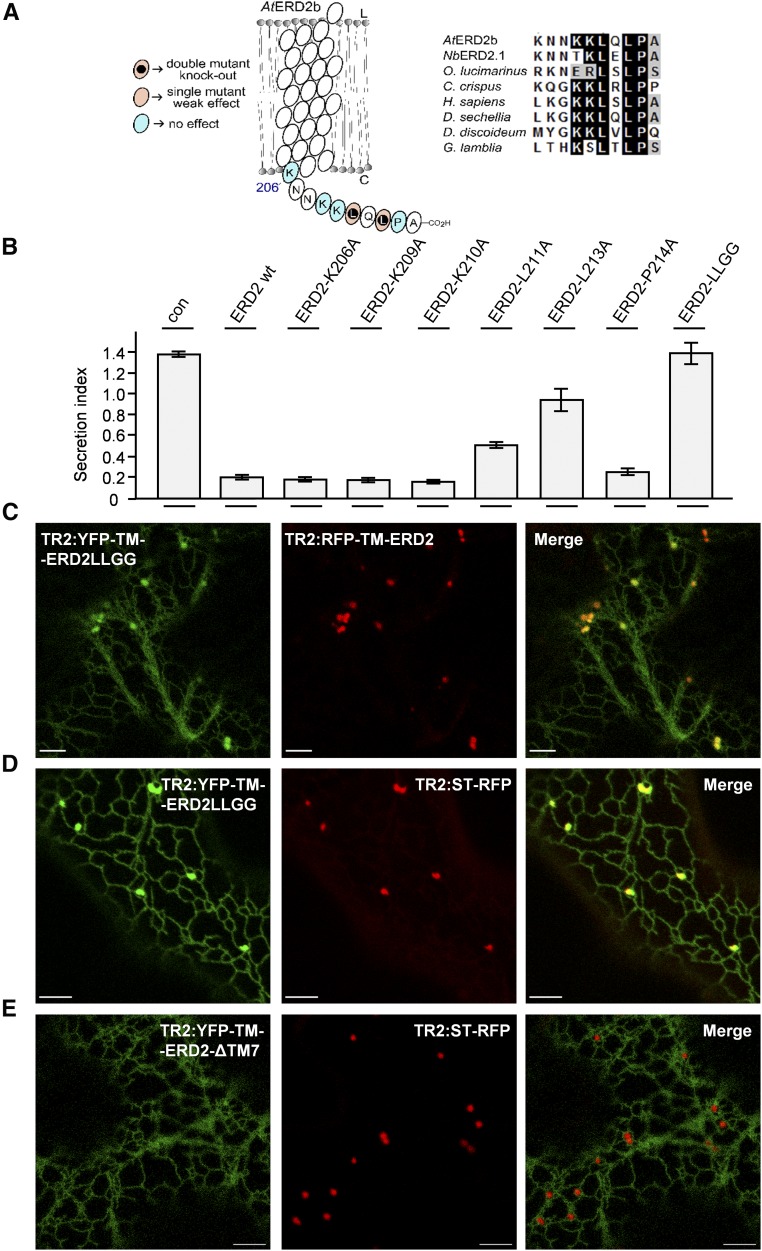
(A) Illustration of point mutagenesis of the C terminus and the observed effects in the biological activity followed by an alignment of ERD2 C termini from different eukaryotes as indicated.
(B) Coexpression of the Amy-HDEL with wild-type ERD2 (wt) and individual alanine replacement mutants in the cytosolic tail of ERD2 in N. benthamiana protoplasts. Fifty micrograms of Amy-HDEL plasmid was cotransfected with 10 μg of effector plasmids. All annotations are as in Figure 1. Mutants that compromise biological activity are identified by increased secretion indices compared with the wild-type ERD2. The double mutant (LLGG) has both conserved leucines (L211 and L213) replaced by the smaller amino acid glycine.
(C) CLSM showing the distribution of YFP-TM-ERD2-LLGG in comparison with RFP-TM-ERD2. Bars = 5 µm.
(D) YFP-TM-ERD2-LLGG in comparison with the Golgi marker ST-RFP. Bars = 5 µm. Notice that the nonfunctional LLGG mutant still reached the Golgi apparatus but was now markedly retained in the ER, similar to the C-terminal fusion ERD2-YFP (see Figure 4C).
(E) Deletion of the last transmembrane domain and cytosolic tail (YFP-TM-ERD2-ΔTM7) caused complete ER retention. Experimental conditions/annotations as in (D).