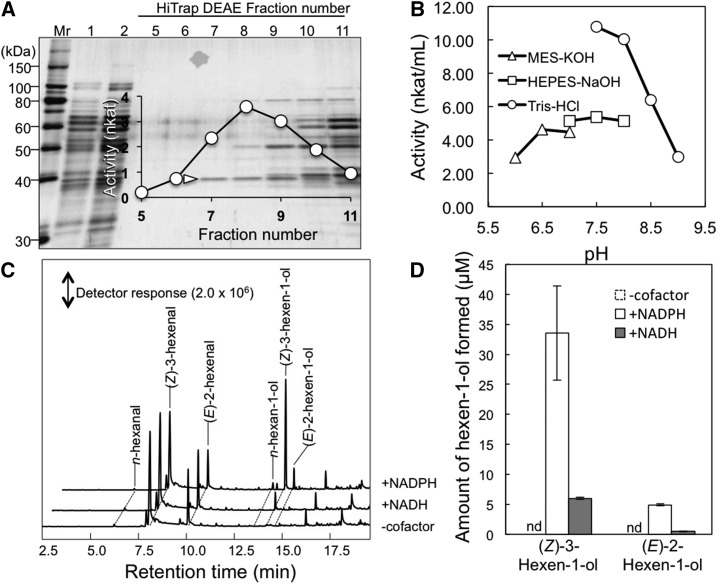
Figure 2.
Purification and properties of hexenal reductase. A, Protein profile of the fractions collected using HiTrap DEAE chromatography. Equal volumes of fractions were loaded onto each lane, and proteins were stained with Coomassie Blue. (Z)-3-Hexenal reductase activities of each fraction (fractions 5–11) are shown. The 38-kD protein used in amino acid sequence analyses is indicated with a white arrowhead. Mr, Molecular mass marker. B, pH activity profile of the partially purified enzyme. C, Gas chromatography-mass spectrometry (GC-MS) chromatograms of products formed from (Z)-3-hexenal in the absence or presence of the cofactor NADH or NADPH. D, Quantities of (Z)-3-hexen-1-ol from C presented as averages ± se (n = 4). Because (Z)-3-hexen-1-ol was not formed in the absence of cofactor, the bars corresponding to activity are indicated by nd, for not detected.