Abstract
Purpose
Oral nitisinone has been shown to increase fur and ocular pigmentation in a mouse model of oculocutaneous albinism (OCA) due to hypomorphic mutations in tyrosinase (TYR), OCA1B. This study determines if nitisinone can improve ocular and/or fur pigmentation in a mouse model of OCA type 3 (OCA3), caused by mutation of the tyrosinase-related protein 1 (Tyrp1) gene.
Methods
Mice homozygous for a null allele in the Tyrp1 gene (C57BL/6J-Tyrp1 b-J/J) were treated with 8 mg/kg nitisinone or vehicle every other day by oral gavage. Changes in fur and ocular melanin pigmentation were monitored. Mature ocular melanosome number and size were quantified in pigmented ocular structures by electron microscopy.
Results
C57BL/6J-Tyrp1 b-J/J mice carry a novel c.403T>A; 404delG mutation in Tyrp1, predicted to result in premature truncation of the TYRP1 protein. Nitisinone treatment resulted in an approximately 7-fold increase in plasma tyrosine concentrations without overt toxicity. After 1 month of treatment, no change in the color of fur or pigmented ocular structures was observed. The distribution of melanosome cross-sectional area was unchanged in ocular tissues. There was no significant difference in the number of pigmented melanosomes in the RPE/choroid of nitisinone-treated and control groups. However, there was a significant difference in the number of pigmented melanosomes in the iris.
Conclusions
Treatment of a mouse model of OCA3 with oral nitisinone did not have a favorable clinical effect on melanin production and minimally affected the number of pigmented melanosomes in the iris stroma. As such, treatment of OCA3 patients with nitisinone is unlikely to be therapeutic.
Keywords: albinism, visual acuity, retinal pigment epithelium
Oculocutaneous albinism (OCA) represents a group of autosomal recessive genetic disorders that result in hypopigmentation of the skin, hair, and eyes.1–3 To date, seven distinct, nonsyndromic forms of recessive OCA have been identified: OCA1, due to mutations in the gene (TYR) encoding the tyrosinase enzyme, TYR; OCA2, associated with mutations in OCA2 (formerly known as the P gene)4,5; OCA3, carrying mutations in the tyrosinase-related protein 1 gene (TYRP1)6; OCA4, due to mutations in the gene encoding solute carrier family 45, member 2 (SLC45A2, formerly known as MATP)7; OCA5, which maps to 4q24, but for which a specific gene has not yet been identified8; OCA6, due to mutations in the solute carrier family 24, member 5 gene (SLC24A5)9–11; and OCA7, associated with mutations in the c10orf11 gene.12 In general, all forms of albinism are either directly or indirectly due to dysfunction of TYR, the first and rate-limiting step in melanin synthesis; indirect causes include abnormal folding of TYR, inefficient targeting of TYR to melanin-containing organelles, the melanosomes, and/or a suboptimal biochemical environment for TYR function in the melanosome.
Albinism results in abnormalities in eye development and visual function: foveal hypoplasia (causing reduced best-corrected visual acuity, including legal blindness or <20/200), abnormal decussation of retinal ganglion cell axons at the optic chiasm, nystagmus, strabismus, photophobia, and refractive errors.13 Currently, there are no treatments beyond supportive measures (e.g., correcting refractive errors, treating amblyopia and strabismus) for the vision deficits in albinism. It is unknown why defects in genes important for melanin synthesis cause developmental eye abnormalities.
OCA3, formerly known as Rufous or (sometimes) Brown albinism, is most prevalent in people of southern African descent,6,14 affecting approximately 1:8500 individuals.15,16 OCA3 patients present with variable degrees of hypopigmentation. Black southern Africans with OCA3 classically have skin color ranging from reddish/bronze to tan/light brown, “ginger” or light brown hair, blue or brown irides, and mild visual impairment. Northern Europeans with OCA3, however, can present somewhat differently: light skin with no ability to tan, yellow-gold hair with orange highlights, and light irides.17 Individuals with OCA3 develop more pigment over time and tend to experience less-severe visual problems than those with other forms of OCA.
OCA3 is caused by mutations in the TYRP1 (often referred to as TRP1) gene located on human chromosome 9p23, which shares 93% homology with its murine counterpart Tyrp1, a product of the brown (b) locus on mouse chromosome 4.6,18–21 TYRP1, as its name implies, shares approximately 40% amino acid homology to TYR, which catalyzes the first and rate-limiting step (tyrosine hydroxylation to DOPA), as well as the second step (DOPA oxidation to DOPA quinone) in melanin production. The enzymatic activity of TYRP1 in melanogenesis is complex; it is likely different in humans versus mice and under different assay conditions (reviewed in Sarangarajan and Boissy22). Mouse TYRP1 exhibits TYR-like activity23,24 in some (but not all) in vitro assays,25 as well as 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity; the latter activity is important in black (eumelanin) production, but not red (pheomelanin) production.26,27
In the melanosome membrane, TYRP1 forms a macromolecular complex with TYR and dopachrome tautomerase (DCT/TYRP2), which regulates TYR enzymatic activity and protein stability.28,29 TYRP1 is synthesized in the endoplasmic reticulum (ER), where it undergoes rapid disulfide bond formation, protein folding, and initial posttranslational glycosylation, assisted by ER chaperones such as the lectin calnexin.30,31 Further glycosylation occurs in the Golgi, before TYRP1 export to the melanosome. Either before or soon after entry into the Golgi, TYRP1 binds to TYR and DCT, to form a melanogenic protein complex. This complex may reduce the cytotoxicity associated with melanin synthesis and/or assist in the folding process by stabilizing the proteins.
The classic brown mutation of Tyrp1 (Tyrp1b) contains the c.618G>A/p.C110Y and c.1266G>A/p.R326H mutations where the cysteine to tyrosine substitution appears to be the key mutagenic change, whereas the arginine to histidine change is probably neutral.32 Mutations in TYRP1/Tyrp1 likely interfere with the process of protein maturation, melanogenic protein complex assembly, and, possibly, enzymatic function. TYRP1 stabilizes TYR and reduction in TYRP1 function results in a decrease in the total amount of TYR activity, without drastically affecting specificity.33–36 Misfolded TYRP1 binds to calnexin longer than normal and also associates with the heat-shock protein BiP.31 In such cases, BiP likely targets TYRP1 for retrograde transport across the ER and eventual degradation in the proteasome.30
We have previously shown that oral administration of the Food and Drug Administration–approved drug nitisinone (NTBC, Orfadin) can increase fur and ocular pigmentation, as well as the number of pigmented melanosomes in ocular tissues of a mouse model of one form of OCA, OCA-1B.37 Nitisinone likely exerts its therapeutic effect by inhibiting 4-hyroxyphenylpyruvate dioxygenase (HPPD), consequently preventing degradation and elevating plasma tyrosine.38 Increased tyrosine stabilizes mutant TYR in the OCA-1B model. In this study, we explored the effect of nitisinone on coat, skin, and eye color, as well as the number and size of melanosomes in the iris, choroid, and retinal pigmented epithelium in a mouse model of OCA3, the brown Jackson mouse C57BL/6J-Tyrp1b-J/b-J.
Materials and Methods
Animal Husbandry and Clinical Examination
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J-Tyrp1b-J/J (JAX stock no. 000068, MGI ID 1855965, C57BL/6J background), BALB/cJ, DBA/2J, and C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed according to our institutional Animal Review Board standards with a 14-hour light, 10-hour dark cycle. Clinical examination and imaging of the anterior segment of the eyes was performed on gently restrained awake mice using a Haag-Streit BQ slit lamp and Imaging Module AM900 software (Bell Ophthalmic Technology, Westville, NJ, USA). After pupil dilation with 1 drop of 1% tropicamide (Alcon Laboratories, Inc., Fort Worth, TX, USA), the posterior segment of the mouse eyes was examined with a 90-D condensing lens (Volk, Mentor, OH, USA). Fundus images were obtained from mice sedated with intraperitoneal injection of 100 mg/mL ketamine and 200 mg/mL xylazine diluted in saline. Images were captured using a Nikon D.90 digital SLR camera with a Nikon 85 mm f/2.8D micro AF-S ED lens (Nikon, Melville, NY, USA) mounted on a custom-made aluminum stand, using a 5-cm-long Hopkins rigid otoscope coupled to a Xenon Nova light source (175 W) and fiber-optic cable (Karl Storz, Segundo, CA, USA). Mice were euthanized with carbon dioxide in compliance with institutional guidelines.
Drug Dosing and Monitoring
Sixteen C57BL/6J-Tyrp1b-J/b-J mice (2 to 3 months old) were designated to receive either nitisinone (NTBC; Swedish Orphan Biovitrum International, SOBI, Stockholm, Sweden), n = 9 or vehicle, n = 7. Nitisinone was dissolved in 50 mM NaHCO3 and brought to a neutral pH before administration. Because hair growth induces melanogenesis, a section of each mouse's coat (2 × 2 cm) was shaved on the right lateral flank before treatment. A dosage of 0.1 to 0.2 mL of drug (8 mg/kg) or vehicle was administered every other day for 1 month via oral gavage. Although this dosage is approximately 4 to 8 times the dosage typically administered to humans with tyrosinemia type 1 (1–2 mg/kg per day), we have previously shown that this dosage is well tolerated in mice and produces maximum plasma tyrosine concentrations of 0.4 to 0.8 mM.37 Increasing the dosage of nitisinone beyond 8 mg/kg in this strain did not result in a significant change in plasma tyrosine (data not shown). Coat color, iris transillumination, and fundus appearance were photodocumented before and after the 1-month treatment period.
Plasma tyrosine was assayed in retro-orbital blood from mice 1 week and 4 weeks into treatment. Based on the amount of blood obtained from nonterminal bleeds, plasma from two mice was pooled to make a single measurement. Plasma samples were frozen on dry ice immediately after collection and stored at −80°C until use. Samples that were ready for assay were gently thawed and diluted with an equal volume of loading buffer (0.2 M lithium citrate, pH 2.2). Samples were then filtered using Vivaspin 500 (3000-Da molecular weight cutoff; Sartorius Stedim Biotech, Goettingen, Germany) and spun in a fixed-angle centrifuge at 14,000g for 60 minutes at 16 to 20°C. The supernatant was collected and tyrosine was quantified on a Biochrom 30 amino acid analyzer (Biochrom, Holliston, MA, USA) using the manufacturer's specifications.
Transmission Electron Microscopy
One eye each from seven treated and seven control mice was dissected and prepared for histology; the second eye was divided into anterior and posterior segments and processed for transmission electron microscopy (TEM). The iris and posterior part of the eyes (choroid, RPE, and retina) were removed and fixed in 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.4, for 12 hours at room temperature. After washing with rinsing buffer (RB; 4% sucrose and 0.15 mM CaCl2 in PB), pH 7.4, at 4°C, the tissues were postfixed in 1% OsO4 in 0.1 M PB, pH 7.4, for 1 hour. Following rinsing and dehydration, the tissues were embedded in Durcupan resin for 72 hours at 60°C. Semi-thin sections (2 μm) were used for tissue orientation. Ultra-thin sections (70–90 nm) were collected in 200-mesh grids and counterstained with 5% uranyl acetate and 0.3% lead citrate. The sections were viewed on a JEOL 1010 EM (JEOL, Peabody, MA, USA) at 60 KV and digital images were acquired at ×12,000 or ×5,000 magnification by AMT software (Advanced Microscopy Techniques, Woburn, MA, USA). The number of pigmented (stage III and IV) melanosomes in the TEM images of treated and untreated mice were counted for each tissue by two masked observers using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). At least three images were analyzed for each tissue for each mouse examined. A darkness threshold of 50 (where 0 is pure black and 255 is pure white) and minimum size of 0.048 μm2 was used to determine adequate pigmentation and size of the particles being counted. The cross-sectional area of each melanosome also was measured using ImageJ. The number of melanosomes per μm2 in each eye structure of each mouse was calculated by dividing the total number of melanosomes counted for a structure by the total measured area of that structure. The average number of melanosomes per μm2 in the treated and untreated groups for each structure was then established by averaging the values of all of the mice in a group.
Genotyping
OCA3, BALB/cJ, DBA/2J, and C57BL/6J mouse eyes were collected in RNAlater (Catalog no. AM7021; Ambion, Austin, TX, USA) after the lenses were removed. RNA/DNA/protein was isolated immediately using the Dr. P-kit per the manufacturer's instructions (Catalog no. K2021010; BioChain, San Diego, CA, USA). Genomic DNA concentration and quality (260/280 ratio >1.8) were estimated by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA). All exons in Tyrp1 gene were PCR amplified and sequenced using the primers listed in Supplementary Table S1.
Western Blotting
Protein concentrations were estimated by Pierce BCA Protein assay kit (Catalog no. 23227; Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein were loaded and separated on 7.5% Criterion TGX precast gels with Tris/Glycine buffer and transferred onto polyvinylidene difluoride membrane with Trans-Blot Turbo RTA transfer kit (Catalog no. 170-4275; Bio-Rad, Hercules, CA, USA). Using iBind Flex (Sigma-Aldrich, St. Louis, MO, USA), the membrane was probed with mouse monoclonal anti-TRP1-23 antibody (1:200; Catalog no. Sc-136388; Santa Cruz Biotechnology, Dallas, TX, USA), mouse monoclonal anti-β-actin antibody clone AC-74 (1:10,000; Catalog no. A228; Thermo Fisher Scientific) and goat anti-mouse 680nm-IR Dye secondary antibody (1:10,000 from Li-COR, Inc., Catalog no. 926–68070). Immunoblots were imaged with Bio-Rad Chemidoc MP imaging system.
Quantitative RT-PCR
RNA sample concentration and quality (260/280 ratio between 1.7 and 2.0) were estimated by Nanodrop. cDNA was synthesized using iSCRIPT Advanced cDNA Synthesis kit (Catalog no. 170-8842; Bio-Rad). Primer combinations covering Tyrp1 exons were used for PCR amplification with SYBR Green PCR Master mix (Catalog no. 4309155; Thermo Fisher Scientific). Relative gene expression (Delta Cq) was calculated on normalization to the mouse housekeeping gene Ppia (Catalog no. 10025636; Bio-Rad).
Results
C57BL/6J-Tyrp1b-J/J Mice Carry a Novel Nonsense Mutation in Tyrp1
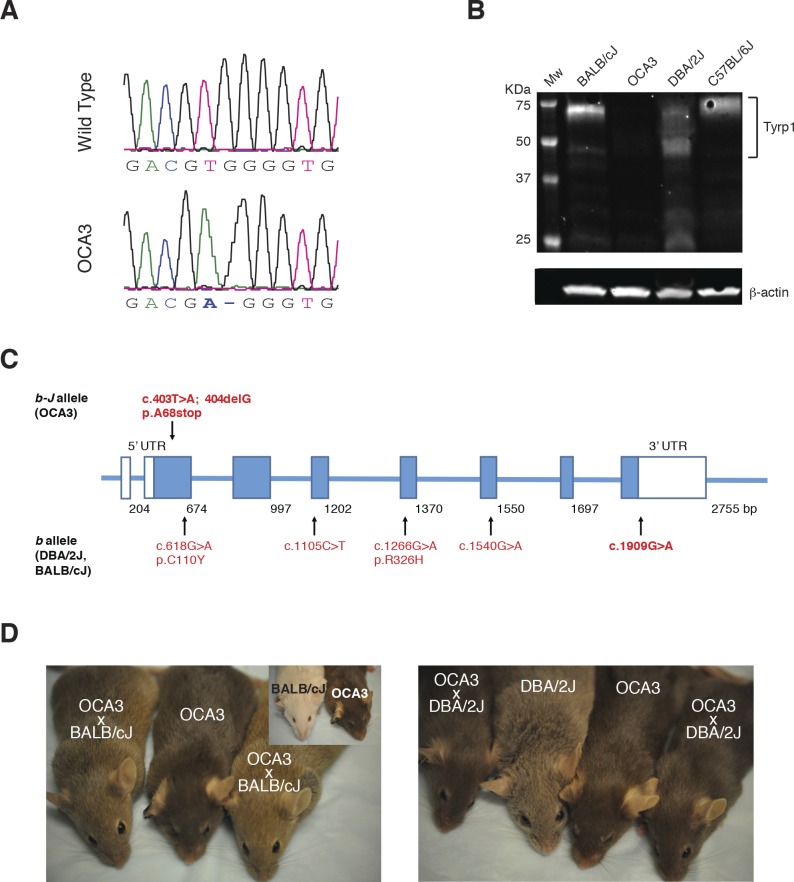
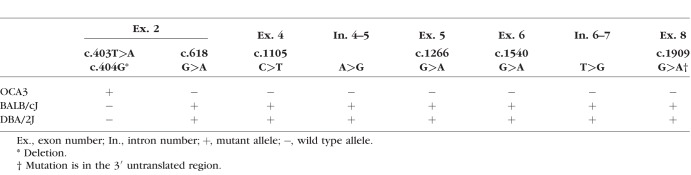
In this study, C57BL/6J-Tyrp1b-J/b-J mice were used as a model for OCA3. Sequencing of tail genomic DNA revealed a novel mutation in Tyrp1 associated with OCA3 in this strain (Fig. 1A). Substitution of the thymine at nucleotide 403 with adenine, followed by a single nucleotide deletion (c.403T>A; 404delG) caused a frameshift, predicted to result in a premature stop codon at amino acid 68. Although full-length mRNA persisted in eye tissue from the mutant mice (Supplementary Table S2), no TYRP1 protein could be detected by Western blotting of ocular protein lysates from C57BL/6J-Tyrp1b-J/b-J (hereafter referred to as OCA3) mice using an antibody to the amino-terminal portion of TYRP1 (Fig. 1B). On the other hand, a protein band of approximately 70 kDa was present in samples from mice carrying Tyrp1b (BALB/cJ and DBA/2J) and wild-type (C57BL/6J) alleles. Figure 1C and the Table compare the alleles detected by sequencing in homozygous OCA3, BALB/cJ, and DBA/2J mice. Gene locations were assigned according to the National Center of Biotechnology Information reference sequence number (NC_000070.6), mouse genome build (GRCm38.p4), variant 1 (Supplementary Table S3). OCA3 mice (dark brown) were crossed with BALB/cJ (albino) and DBA/2J (brown) mice. The color of the resulting offspring was a light brown for OCA3 × BALB/cJ (obligate Tyrc/+, Tyrp1b/b-J) and dark brown for OCA3 × DBA/2J (obligate Tyrp1b/b-J, GpnmbR150X/+, Myo5ad/+) (Fig. 1D). This suggests that the null brown Jackson (b-J) allele may synergize with a single mutant Tyr allele and/or interacts in a complex way with the missense Tyrpb allele to produce a lighter coat color. The latter seems less likely, given that the DBA/2J × OCA3 offspring have the same Tyrpb/b-J allele combination and a coat color similar to the parent OCA3 strain. Because mutation of Tyrp1 in the DBA/2J mouse strain is associated with iris stromal atrophy and pigmentary glaucoma, we examined three representative OCA3 mice at approximately 11 months of age39; these mice did not show any evidence of pigment dispersion in the anterior segment, iris atrophy, or clinical evidence of glaucoma, such as corneal ectasia (data not shown).
Figure 1.
Novel Tyrp1 mutation in C57BL/6J-Tyrp1b-J/J mice. DNA sequencing detected c.403T>A; 404delG (p.A68stop) mutation, predicted to result in premature truncation (A). Western blot of protein lysates from whole eyes of control C57BL/6J mice (Tyrp1 wild-type allele), BALB/cJ and DBA/2J (Tyrp1b/b allele), and OCA3 (Tyrp1b-J/b-J allele) mutants (B). A 70-kDa, presumably glycosylated, band was detected in control, BALB/cJ, and DBA/2J lanes. At 50 kDa, an additional, presumably nonglycosylated, band of variable intensity was detected only in BALB/cJ and DBA/2J b allele samples. In addition, lower molecular weight bands were visible in the DBA/2J sample. No bands were visible in the OCA3 lane. Schematic representation of Tyrp1 b-J and b alleles (C). Color coat of parent and offspring from crossing of OCA3 with BALB/cJ and DBA/2J mutants (D).
Table.
Mutation Analysis of the b Locus in OCA3, BALB/cJ, and DBA/2J Mutant Mice
Nitisinone Increases Plasma Tyrosine But Does Not Increase Coat or Ocular Pigmentation in a Mouse Model of OCA3
A total of nine mice (2 to 3 months old) were treated with 8 mg/kg of nitisinone via oral gavage every other day for 1 month; seven age-matched controls were fed vehicle in an identical fashion. To allow for easy comparison of coat color and to stimulate melanin production in growing fur, a 2 × 2-cm section of each mouse's coat was shaved from the right flank before treatment.
To evaluate how effectively the drug elevated tyrosine levels, plasma tyrosine was assayed from retro-orbital blood obtained from nonterminal bleeds. Treatment with nitisinone increased tyrosine levels nearly 7-fold, resulting in an average concentration of 553 μM in the treated group compared with 80 μM in the control group, and much higher than the baseline tyrosine plasma content in other Tyr mutant mouse strains such as Tyrc-h/c-h (74–99 μM) and Tyrc-2J/c-2J (109–139 μM).37 Even though plasma tyrosine was substantially elevated, no discernible side effects were observed. The treated mice did not exhibit abnormal behavior or develop gross skin or corneal lesions, which can be side effects of hypertyrosinemia in humans.
Physical examination revealed that areas of new hair growth did not show an increase in pigmentation (Figs. 2A, 2B). Similarly, slit lamp examination of the anterior segment showed that the irides of the treated and control groups had comparable pigmentation based on iris transillumination (Figs. 2C, 2D). Dilated funduscope examination showed no observable difference in pigmentation between the two groups (Figs. 2E, 2F). Likewise, hematoxylin-eosin staining of the iris, RPE, and choroid of one eye each of treated mice (n = 7) did not show any discernible difference in melanin pigmentation, compared with controls (n = 7 mice, data not shown).
Figure 2.
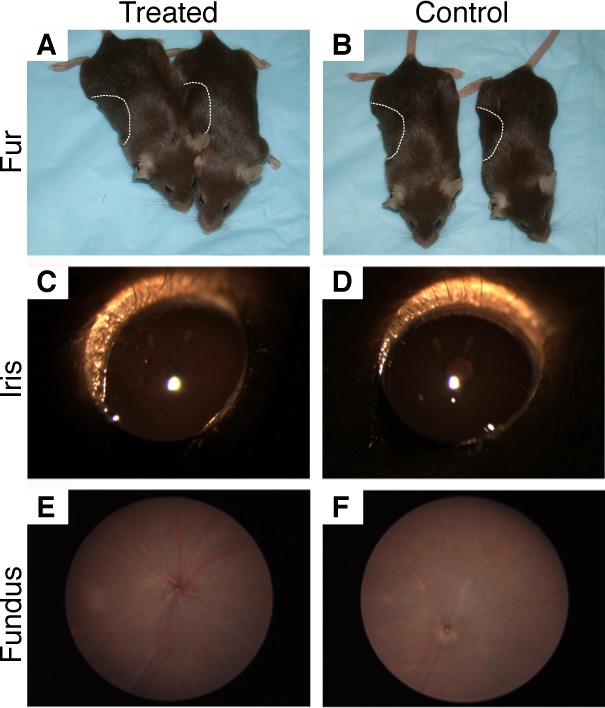
Treatment of mice with 8 mg/kg oral nitisinone for 1 month did not visibly increase fur (A, B), iris (C, D), or fundus (E, F) pigmentation. The dotted lines in (A) and (B) outline the patch of fur that was shaved, as pigment is preferentially deposited in actively growing fur.
Nitisinone Has Minimal Effects on Melanosome Size or Density in Ocular Tissues
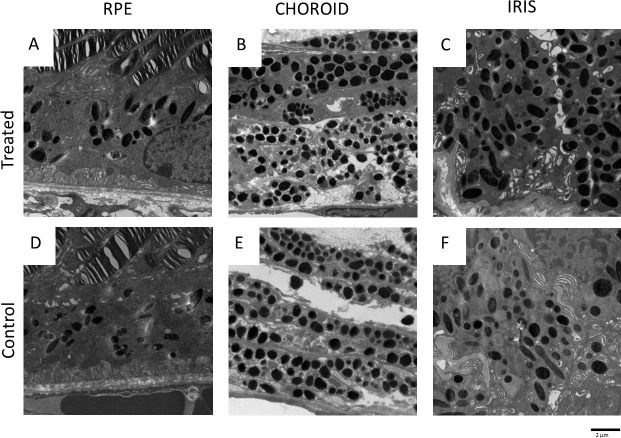
To quantify the effect of nitisinone on the number and size of melanosomes in ocular tissue, we performed TEM of the iris, choroid, and RPE (one eye each from n = 7 treated and n = 7 control mice) (Fig. 3). On processing of the images using ImageJ, and analysis of the data using two-tailed t-test, we found no statistically significant difference between the number of melanosomes in the RPE and choroid of treated compared with control mice (Fig. 4). However, we detected a statistically significant difference in the number of pigmented melanosomes in the iris stroma of the treated mice compared with untreated (Fig. 4). The cross-sectional area of melanosomes did not differ significantly between the two groups (Fig. 5). Melanosomes from all ocular tissues were between 0.048 and 0.8 μm2 in size and comparable to previously published melanosome size in choroid (0.07 ± 0.04 μm2) and RPE (0.12 ± 0.08 μm2) of wild-type C57BL/6J mice.40 These data demonstrate that treatment with nitisinone does not have a major impact on melanin pigmentation at either the gross or microscopic level in the C57BL/6J-Tyrp1b-J/b-J mouse model of OCA3.
Figure 3.
Electron micrographs of the melanin-containing ocular structures. RPE (A, D), choroid (B, E), and iris (C, F) are shown.
Figure 4.
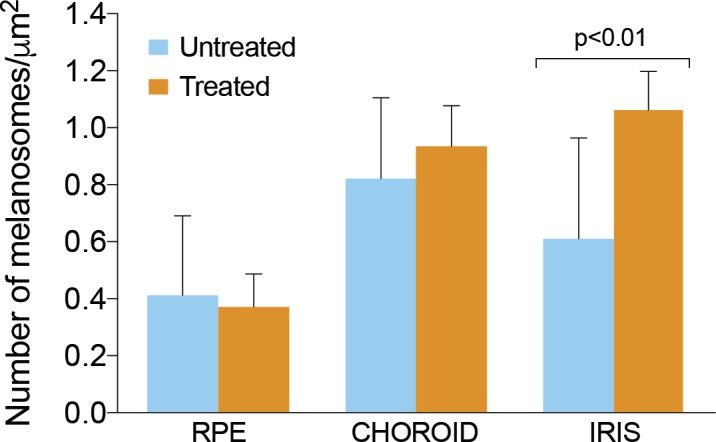
Quantification of the number of pigmented melanosomes in the iris, choroid, and RPE of treated (n = 7) and control C57BL/6J-Tyrp1b-J/J mice (n = 7). In the iris, there was a statistically significant increase in the number of pigmented melanosomes, two-tailed t-test P < 0.01. Error bars represent ± SD.
Figure 5.
Distribution of melanosome cross-sectional area in treated and control C57BL/6J-Tyrp1b-J/J mice. There was no significant difference between the two groups, although melanosomes from the untreated mice covered a larger size range.
Discussion
We have previously demonstrated that treatment with nitisinone increases coat and ocular (coat>ocular) pigmentation in a mouse model of OCA-1B.37 Elevated tyrosine levels stabilize mutant TYR, improving its enzymatic function and increasing the number of mature melanosomes in ocular tissue. A pilot clinical study (NCT01838655) in adult patients with OCA-1B is currently being conducted to determine if nitisinone has a similar effect in humans, and if an increase in pigmentation can improve visual function.
The positive effect that nitisinone has on pigmentation in an OCA-1B mouse model suggests that the drug may increase pigmentation in mouse models of other forms of OCA. TYRP1 forms a melanogenic protein complex with TYR and is involved in its regulation and stabilization. Thus, we postulated that stabilizing TYR with elevated plasma tyrosine would produce a similar effect in a mouse model of OCA3. We reasoned that increased plasma tyrosine might result in improved TYR function, possibly by binding to the protein and perhaps chaperoning its proper folding and targeting.
Both humans and mice display a range of mutations in the TYRP1/Tyrp1 gene, including nonsense and missense.14,17,22,41–45 In a group of TYRP1 patients, only compound nonsense mutation on one allele and missense mutation on the other allele result in disease.17,42 Furthermore, TYRP1 variants are associated with pigment-related phenotypes distinct from albinism per se.46 The classic brown Tyrp1b/b mouse is homozygous for the c.618G>A/p.C110Y and c.1266G>A/p.R326H mutations in the Tyrp1 gene (NM_031202.3)32 that disrupt TYRP1 protein function, reducing the amount of eumelanin production.21 In contrast, the b-J allele carried by the C57BL/6J-Tyrp1b-J/J (JAX stock no. 000068) mouse presented novel c.403T>A mutation and 404delG that introduced a stop codon and resulted in undetectable TYRP1 protein (null). This animal model recapitulates OCA3 human mutations that result in loss of TYRP1 protein.14,47
Because this strain of mice is substantially more pigmented than other mouse models of more severe forms of albinism, we were looking for small changes in response to nitisinone treatment. Nitisinone administration increased plasma tyrosine in the C57BL/6J-Tyrp1b-J/b-J mouse model without causing any discernible physical or behavioral side effects. However, this did not result in an overt increase in coat and ocular pigmentation or an effect on melanosome size; pigmented melanosome number was increased mildly only in iris tissue and was unchanged in RPE and choroid. Our results do not exclude the possibility that nitisinone might exert a therapeutic effect in the setting of a different mutation, in which TYRP1 protein is present, albeit dysfunctional or not fully functional, such is the case for DBA/2J mice. In mice, the Tyrp1b/b allele causes iris stromal atrophy39 and other mutations in Tyrp1 result in hypopigmentation and melanocyte loss, presumably through a dominant, toxic gain-of-function mechanism.21 Notably, mice homozygous or compound heterozygous for the b and b-J alleles display different coat colors depending on the context of the Tyr c allele, underscoring the complex nature of the genetic interactions between Tyrp1, Tyr, and potentially other genes important in melanogenesis.
This study chose one dose of nitisinone (8 mg/kg), based on its ability to maximally raise plasma tyrosine in mice. Because the active pool of tyrosine for stabilizing proteins is within the cell, we cannot rule out that higher doses of drug might have produced an effect. An additional limitation in this study is its comparatively small sample size, which could mask a small, but statistically significant effect. However, because our ultimate goal was to determine if nitisinone is clinically effective in treating human patients with OCA3, we felt that only robust results would meet our criteria for translating our findings into a clinical trial. We conclude that our data do not justify the treatment with nitisinone of albinism patients carrying homozygous nonsense mutations in OCA3. We anticipate that our current pilot clinical trial of nitisinone in patients with OCA-1B will inform the use of nitisinone in that and other forms of albinism.
Supplementary Material
Acknowledgments
The authors thank Robert Hufnagel for fruitful discussions, William Gahl and SOBI International (Stockholm, Sweden) for providing the nitisinone for this study, and Anne Warburton for technical assistance.
Supported by the intramural program of the National Eye Institute, National Institutes of Health, U.S. Department of Health and Human Services.
Disclosure: I.F. Onojafe, None; L.H. Megan, None; M.G. Melch, None; J.O. Aderemi, None; R.P. Alur, None; M.S. Abu-Asab, None; C.-C. Chan, None; I.M. Bernardini, None; J.S. Albert, None; T. Cogliati, None; D.R. Adams, None; B.P. Brooks, None
References
- 1.King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism 8th ed. New York: McGraw-Hill;; 2001. pp. 5587–5627. [Google Scholar]
- 2.Simeonov DR, Wang X, Wang C, et al. DNA variations in oculocutaneous albinism: an updated mutation list and current outstanding issues in molecular diagnostics. Hum Mutat. 2013;34:827–835. doi: 10.1002/humu.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montoliu L, Gronskov K, Wei AH, et al. Increasing the complexity: new genes and new types of albinism. Pigment Cell Melanoma Res. 2014;27:11–18. doi: 10.1111/pcmr.12167. [DOI] [PubMed] [Google Scholar]
- 4.Durham-Pierre D, Gardner JM, Nakatsu Y, et al. African origin of an intragenic deletion of the human P gene in tyrosinase positive oculocutaneous albinism. Nat Genet. 1994;7:176–179. doi: 10.1038/ng0694-176. [DOI] [PubMed] [Google Scholar]
- 5.Rosemblat S, Durham-Pierre D, Gardner JM, Nakatsu Y, Brilliant MH, Orlow SJ. Identification of a melanosomal membrane protein encoded by the pink-eyed dilution (type II oculocutaneous albinism) gene. Proc Natl Acad Sci U S A. 1994;91:12071–12075. doi: 10.1073/pnas.91.25.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boissy RE, Zhao H, Oetting WS, et al. Mutation in and lack of expression of tyrosinase-related protein-1 (TRP-1) in melanocytes from an individual with brown oculocutaneous albinism: a new subtype of albinism classified as “OCA3.”. Am J Hum Genet. 1996;58:1145–1156. [PMC free article] [PubMed] [Google Scholar]
- 7.Newton JM, Cohen-Barak O, Hagiwara N, et al. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum Genet. 2001;69:981–988. doi: 10.1086/324340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kausar T, Bhatti MA, Ali M, Shaikh RS, Ahmed ZM. OCA5, a novel locus for non-syndromic oculocutaneous albinism, maps to chromosome 4q24. Clin Genet. 2013;84:91–93. doi: 10.1111/cge.12019. [DOI] [PubMed] [Google Scholar]
- 9.Lamason RL, Mohideen MAPK, Mest JR, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 10.Mondal M, Sengupta M, Samanta S, Sil A, Ray K. Molecular basis of albinism in India: evaluation of seven potential candidate genes and some new findings. Gene. 2012;511:470–474. doi: 10.1016/j.gene.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Wei AH, Zang DJ, Zhang Z, et al. Exome sequencing identifies SLC24A5 as a candidate gene for nonsyndromic oculocutaneous albinism. J Invest Dermatol. 2013;133:1834–1840. doi: 10.1038/jid.2013.49. [DOI] [PubMed] [Google Scholar]
- 12.Gronskov K, Dooley CM, Ostergaard E, et al. Mutations in c10orf11, a melanocyte-differentiation gene, cause autosomal-recessive albinism. Am J Hum Genet. 2013;92:415–421. doi: 10.1016/j.ajhg.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summers CG. Vision in albinism. Trans Am Ophthalmol Soc. 1996;94:1095–1155. [PMC free article] [PubMed] [Google Scholar]
- 14.Manga P, Kromberg JG, Box NF, Sturm RA, Jenkins T, Ramsay M. Rufous oculocutaneous albinism in southern African Blacks is caused by mutations in the TYRP1 gene. Am J Hum Genet. 1997;61:1095–1101. doi: 10.1086/301603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kromberg JG, Castle DJ, Zwane EM, et al. Red or rufous albinism in southern Africa. Ophthalmic Paediatr Genet. 1990;11:229–235. doi: 10.3109/13816819009020984. [DOI] [PubMed] [Google Scholar]
- 16.Gronskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis. 2007;2:43. doi: 10.1186/1750-1172-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rooryck C, Roudaut C, Robine E, Musebeck J, Arveiler B. Oculocutaneous albinism with TYRP1 gene mutations in a Caucasian patient. Pigment Cell Res. 2006;19:239–242. doi: 10.1111/j.1600-0749.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 18.Jackson IJ. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988;85:4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murty VV, Bouchard B, Mathew S, Vijayasaradhi S, Houghton AN. Assignment of the human TYRP (brown) locus to chromosome region 9p23 by nonradioactive in situ hybridization. Genomics. 1992;13:227–229. doi: 10.1016/0888-7543(92)90228-k. [DOI] [PubMed] [Google Scholar]
- 20.Chintamaneni CD, Ramsay M, Colman MA, Fox MF, Pickard RT, Kwon BS. Mapping the human CAS2 gene, the homologue of the mouse brown (b) locus, to human chromosome 9p22-pter. Biochem Biophys Research Commun. 1991;178:227–235. doi: 10.1016/0006-291x(91)91803-k. [DOI] [PubMed] [Google Scholar]
- 21.Jackson I, Chambers D, Rinchik E, Bennett D. Characterization of TRP-1 mRNA levels in dominant and recessive mutations at the mouse brown (b) locus. Genetics. 1990;126:451–459. doi: 10.1093/genetics/126.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarangarajan R, Boissy RE. Tyrp1 and oculocutaneous albinism type 3. Pigment Cell Res. 2001;14:437–444. doi: 10.1034/j.1600-0749.2001.140603.x. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez M, Tsukamoto K, Hearing VJ. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991;266:1147–1156. [PubMed] [Google Scholar]
- 24.Jimenez-Cervantes C, Garcia-Borron JC, Valverde P, Solano F, Lozano JA. Tyrosinase isoenzymes in mammalian melanocytes. 1. Biochemical characterization of two melanosomal tyrosinases from B16 mouse melanoma. Eur J Biochem. 1993;217:549–556. doi: 10.1111/j.1432-1033.1993.tb18276.x. [DOI] [PubMed] [Google Scholar]
- 25.Sarangarajan R, Zhao Y, Babcock G, Cornelius J, Lamoreux ML, Boissy RE. Mutant alleles at the brown locus encoding tyrosinase-related protein-1 (TRP-1) affect proliferation of mouse melanocytes in culture. Pigment Cell Res. 2000;13:337–344. doi: 10.1034/j.1600-0749.2000.130506.x. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, Urabe K, Winder A, et al. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994;13:5818–5825. doi: 10.1002/j.1460-2075.1994.tb06925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiménez-Cervantes C, Solano F, Kobayashi T, et al. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1) J Biol Chem. 1994;269:17993–18000. [PubMed] [Google Scholar]
- 28.Orlow S, Zhou B, Chakraborty A, Drucker M, Pifko-Hirst S, Pawelek J. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol. 1994;103:196–201. doi: 10.1111/1523-1747.ep12392743. [DOI] [PubMed] [Google Scholar]
- 29.Hearing V, Tsukamoto K, Urabe K, Kameyama K, Montague P, Jackson I. Functional properties of cloned melanogenic proteins. Pigment Cell Res. 1992;5:264–270. doi: 10.1111/j.1600-0749.1992.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 30.Branza-Nichita N, Petrescu AJ, Negroiu G, Dwek RA, Petrescu SM. N-glycosylation processing and glycoprotein folding—Lessons from the tyrosinase-related proteins. Chem Rev. 2000;100:4697–4711. doi: 10.1021/cr990291y. [DOI] [PubMed] [Google Scholar]
- 31.Negroiu G, Dwek RA, Petrescu SM. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J Biol Chem. 2000;275:32200–32207. doi: 10.1074/jbc.M005186200. [DOI] [PubMed] [Google Scholar]
- 32.Zdarsky E, Favor J, Jackson IJ. The molecular-basis of Brown, an Old Mouse mutation, and of an induced revertant to wild-type. Genetics. 1990;126:443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao HQ, Eling DJ, Medrano EE, Boissy RE. Retroviral infection with human tyrosinase-related protein-1 (TRP-1) cDNA upregulates tyrosinase activity and melanin synthesis in a TRP-1-deficient melanoma cell line. J Invest Dermatol. 1996;106:744–752. doi: 10.1111/1523-1747.ep12345799. [DOI] [PubMed] [Google Scholar]
- 34.Winder AJ, Odh G, Rosengren E, Rorsman H. Fibroblasts co-expressing tyrosinase and the B-protein synthesize both eumelanin and pheomelanin. Biochim Biophys Acta. 1995;1268:300–310. doi: 10.1016/0167-4889(95)00089-b. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Imokawa G, Bennett DC, Hearing VJ. Tyrosinase stabilization by Tyrp1 (the brown locus protein) J Biol Chem. 1998;273:31801–31805. doi: 10.1074/jbc.273.48.31801. [DOI] [PubMed] [Google Scholar]
- 36.Manga P, Sato K, Ye L, Beermann F, Lamoreux ML, Orlow SJ. Mutational analysis of the modulation of tyrosinase by tyrosinase-related proteins 1 and 2 in vitro. Pigment Cell Res. 2000;13:364–374. doi: 10.1034/j.1600-0749.2000.130510.x. [DOI] [PubMed] [Google Scholar]
- 37.Onojafe IF, Adams DR, Simeonov DR, et al. Nitisinone improves eye and skin pigmentation defects in a mouse model of oculocutaneous albinism. J Clin Invest. 2011;121:3914–3923. doi: 10.1172/JCI59372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lock EA, Ellis MK, Gaskin P, et al. From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis. 1998;21:498–506. doi: 10.1023/a:1005458703363. [DOI] [PubMed] [Google Scholar]
- 39.Anderson MG, Smith RS, Hawes NL, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 40.Brooks BP, Larson DM, Chan CC, et al. Analysis of ocular hypopigmentation in Rab38cht/cht mice. Invest Ophthalmol Vis Sci. 2007;48:3905–3913. doi: 10.1167/iovs.06-l464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oetting WS, King RA. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat. 1999;13:99–115. doi: 10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 42.Zhang KH, Li Z, Lei J, et al. Oculocutaneous albinism type 3 (OCA3): analysis of two novel mutations in TYRP1 gene in two Chinese patients. Cell Biochem Biophys. 2011;61:523–529. doi: 10.1007/s12013-011-9234-0. [DOI] [PubMed] [Google Scholar]
- 43.Sarangarajan R, Budev A, Zhao Y, Gahl WA, Boissy RE. Abnormal translocation of tyrosinase and tyrosinase-related protein 1 in cutaneous melanocytes of Hermansky-Pudlak Syndrome and in melanoma cells transfected with anti-sense HPS1 cDNA. J Invest Dermatol. 2001;117:641–646. doi: 10.1046/j.0022-202x.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 44.Chiang PW, Spector E, Scheuerle A. A case of Asian Indian OCA3 patient. Am J Med Genet A. 2009;149A:1578–1580. doi: 10.1002/ajmg.a.32930. [DOI] [PubMed] [Google Scholar]
- 45.Forshew T, Khaliq S, Tee L, et al. Identification of novel TYR and TYRP1 mutations in oculocutaneous albinism. Clin Genet. 2005;68:182–184. doi: 10.1111/j.1399-0004.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 46.Kenny EE, Timpson NJ, Sikora M, et al. Melanesian blond hair is caused by an amino acid change in TYRP1. Science. 2012;336:554. doi: 10.1126/science.1217849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada M, Sakai K, Hayashi M, et al. Oculocutaneous albinism type 3: a Japanese girl with novel mutations in TYRP1 gene. J Dermatol Sci. 2011;64:217–222. doi: 10.1016/j.jdermsci.2011.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.