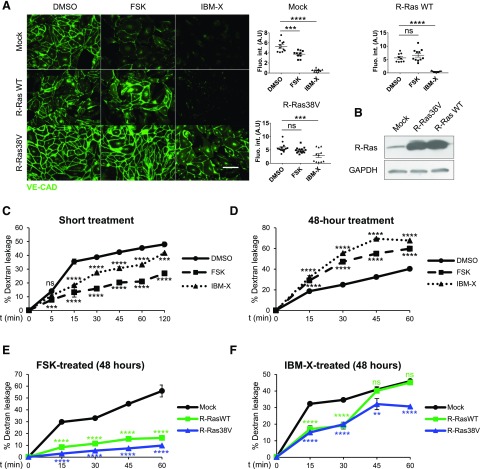
Figure 5.
Forced expression of R-Ras restores endothelial barrier in ECs with elevated cAMP. A) Confluent monolayer of HUVECs transduced with mock, WT R-Ras (WT), or R-Ras38V were treated with DMSO, forskolin, or IBMX for 48 h and stained for VE-cadherin to assess junctional integrity. Integrated intensity of fluorescence signal was quantified in 3 distinct areas from at least 4 micrographs for each condition by ImageJ software. Scale bar, 100 µm. Ns, not significant. *P < 0.05, ***P < 0.001, ****P < 0.0001 (1-way ANOVA). B) R-Ras expression in HUVECs was analyzed by Western blot 4 d after transduction with mock, R-Ras38V, or R-Ras WT lentivirus. C, D) Endothelial permeability was assessed shortly after cAMP elevation (C) or in response to prolonged cAMP signaling (D). HUVECs were cultured in 24-well Transwell chambers (0.4 μm pore size) for 48 h to produce confluent monolayer. For short-term stimulation, cells were treated with DMSO, forskolin, or IBMX (t = 0 min), and FITC-dextran (70 kDa, 1 mg/ml) was added simultaneously into upper chamber. Fluorescence signal in lower compartment was measured by fluorophotometer at different time points (5–120 min after treatment). For long-term stimulation, permeability of HUVEC monolayer was determined after 48 h of cell incubation with DMSO, forskolin, or IBMX. FITC-dextran (70 kDa, 1 mg/ml) was subsequently added to upper chamber. Fluorescence signal in lower compartment was measured by fluorophotometer at different time points. Empty chambers without cells were used as reference (100% dextran leakage) for percentage calculation. E, F) Prolonged effects of cAMP signaling were analyzed using ECs transduced with mock, WT R-Ras, or R-Ras38V in forskolin- (E) or IBMX- (F) treated culture. Ns, not significant. **P < 0.01, ***P < 0.001, ****P < 0.0001 (2-way ANOVA).