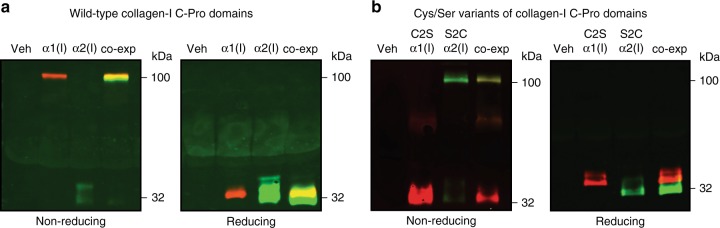
Fig. 3.
The presence or absence of a single cysteine residue defines the capacity of collagen-I C-Pro domains to form stable, disulfide-linked homotrimers. a Immunoblot analysis of individually expressed wild-type, HA-tagged C-Proα1(I) (red) and wild-type, FLAG-tagged C-Proα2(I) (green) proteins under non-reducing and reducing conditions showing that wild-type C-Proα1(I) forms a disulfide-linked homotrimer, whereas wild-type C-Proα2(I) does not, recapitulating the known disulfide-dependent assembly patterns of full-length Colα1(I) and Colα2(I). Co-expression of wild-type C-Proα1(I) and wild-type C-Proα2(I) rescues wild-type C-Proα2(I) into a heterotrimer, as shown by the yellow color indicating red and green overlap. b Immunoblot analysis of individually expressed HA-tagged Cys1265Ser (C2S) C-Proα1(I) (red) and FLAG-tagged Ser1169Cys (S2C) C-Proα2(I) (green) proteins under non-reducing and reducing conditions showing that the serine variant of C-Proα1(I) is no longer able to form a disulfide-linked homotrimer. In contrast, the cysteine variant of C-Proα2(I) is able to form disulfide-linked homotrimers. Co-expression of C2S C-Proα1(I) and S2C C-Proα2(I) rescues C2S C-Proα1(I) into a heterotrimer, as shown by the yellow color indicating red and green overlap