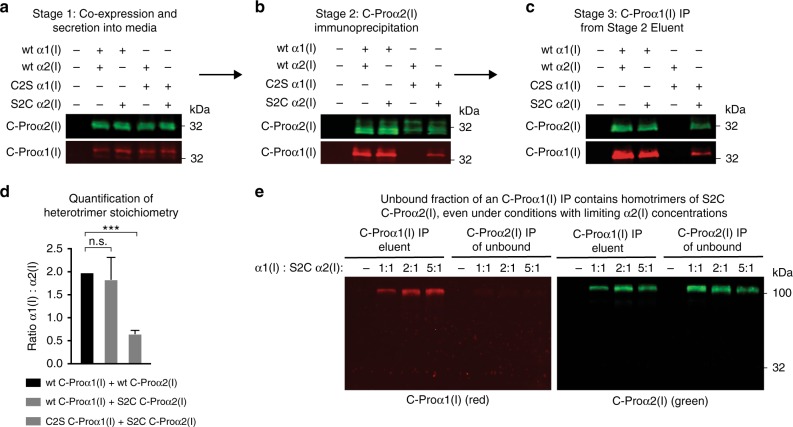
Fig. 5.
Co-expression analysis of heterotrimeric assembly products. This figure is supplemented with arrows from a to b and from b to c to indicate that the samples generated from each stage were then used in the subsequent analysis. Both wild-type and C2S C-Proα1(I) were tagged with HA. Both wild-type and S2C C-Proα2(I) were tagged with FLAG. All C-Proα1(I) signal was detected using an antibody raised against C-Proα1(I) (shown in red). All C-Proα2(I) signal was detected using an antibody raised against the FLAG epitope (shown in green). a Stage 1: Media harvested from cells transfected with the indicated combinations of C-Proα1(I) and C-Proα2(I) was immunoblotted for the presence of each construct on a reducing SDS-PAGE gel. b Stage 2: Immunoprecipitation of C-Proα2(I) from the Stage 1 media samples (a) under non-reducing, Ca2+-depleted conditions. Immunoprecipitated samples were immunoblotted for the presence of each construct on a reducing SDS-PAGE gel. c Stage 3: Final step in the purification of heterotrimers via immunoprecipitation of C-Proα1(I) from the eluent obtained in Stage 2 (b). Immunoprecipitated samples were immunoblotted for the presence of each construct on a reducing SDS-PAGE gel. d Quantification of the C-Proα1(I):C-Proα2(I) ratio of the purified heterotrimers obtained in Stage 3 (c). The bar chart shows the average ratio across four biological replicates. ***p value <0.001 as determined by a t test. Error bars indicate standard deviation from the mean for each sample. e Co-immunoprecipitation experiments on heterotrimers formed when wild-type C-Proα1(I) and S2C C-Proα2(I) were co-expressed at ratios of 1:1, 2:1, and 5:1. The conditioned media were first subjected to an HA immunoprecipitation to extract all C-Proα1(I)-containing species, and the supernatant (unbound fraction) was then subjected to a FLAG immunoprecipitation to purify any remaining S2C C-Proα2(I). Elutions from the FLAG immunoprecipitation were analyzed on a non-reducing, SDS-PAGE gel. n.s. = not significant