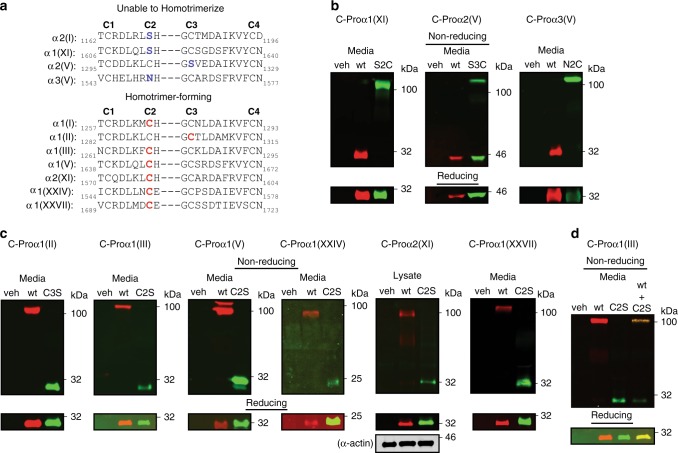
Fig. 7.
The cysteine-based code for collagen C-Pro assembly is generalizable across the fibrillar collagens. a Alignment of the interstrand disulfide-bonding region of the human fibrillar collagen C-propeptides highlights that C-Pro domains known to homotrimerize contain all four conserved cysteine residues, whereas C-Pro domains known to only form heterotrimers lack a single cysteine at C2 or C3. The residue colored red in each protein sequence corresponds to the mutated residue analyzed in b, c. Amino acid numbering was derived from the corresponding full-length procollagens. b Immunoblot analyses of individually expressed wild-type fibrillar collagen C-Pro domains known to only form heterotrimers. Assembly was analyzed under non-reducing and reducing conditions. Wild-type variants (HA-tagged; red) all migrated as monomers, while all variants in which the missing cysteine residue was re-introduced (FLAG-tagged; green) gained the ability to homotrimerize in a disulfide-dependent manner. c Immunoblot analysis of individually expressed wild-type fibrillar collagen C-Pro domains known to have the capacity to homotrimerize. Assembly was analyzed under non-reducing and reducing conditions. Wild-type variants (HA-tagged; red) all migrated as disulfide-dependent homotrimers, while variants in which a single cysteine residue was mutated to serine (FLAG-tagged; green) lost the capacity to form disulfide-linked homotrimers. Note that C-Proα2(XI) was not secreted and was detected only in cell lysate. d Co-expression of the wild-type C-Proα1(III) domain with the C2S C-Proα1(III) demonstrates the wild-type protein’s ability to rescue the monomeric C2S variant into a disulfide-linked trimer, shown by the overlap of green and red signal (yellow)