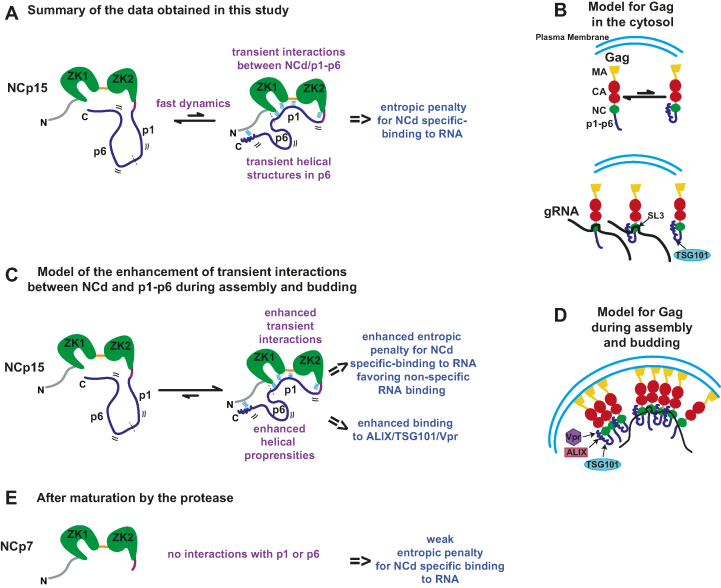
Figure 4.
Summary of the dynamic behaviour and RNA-binding properties observed for the C-terminal part of Gag (NCp15) and implications for its binding properties during virion genesis. (A) The fast dynamics motions observed in NCp15 are characterized by transient interactions between NCd and p1–p6 and transient helical folding of p6. These dynamical processes result in an entropic penalty for NCd specific-binding to RNA. (B) In the context of Gag in the cytosol, the C-terminal part of Gag is in equilibrium between the two forms, that can bind equally SL3 and the presence of helical folding can also promote binding to TSG101. (C) Transient interactions could be amplified during assembly and budding of virions due to the packing and oligomerization of Gag to the plasma membrane. The enhancement of the transient interactions between NCd and p1–p6 and the enhancement of helical propensities of p6 could have two non-related consequences: 1) an enhanced entropic penalty for NCd specific-binding to RNA that could prevent the binding of NCd to its preferred sites and favor its binding to non-specific sequences explaining previous results obtained in cell and 2) enhanced binding to partners of p6. We propose that this enhanced entropic penalty explains the specificity change observed for Gag/RNA interaction occuring in immature virion. (D) Model for Gag during assembly and budding taking into account hypothesis of (C). (E) After maturation by the protease, NCp7 is freed from p1 and p6 and from Gag oligomerization and can again binds to its specific RNA sequences.