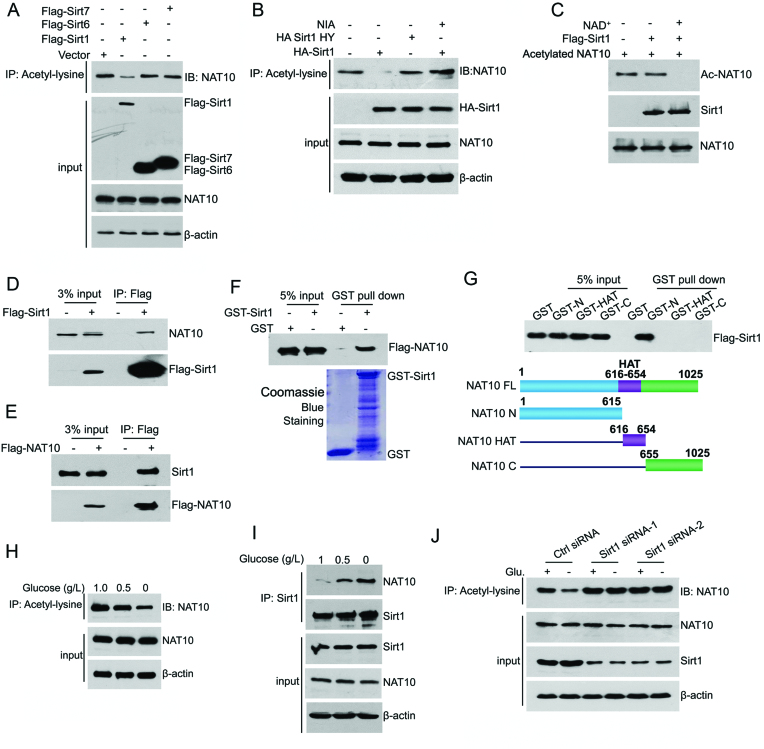
Figure 1.
NAT10 is deacetylated by Sirt1 upon energy deprivation. (A) HCT116 cells were transfected with the indicated plasmids. Cell lysates were prepared and subjected to immunoprecipitation using an anti-acetyl-lysine antibody. The acetylation levels of NAT10 in the immunoprecipitants were evaluated by western blot using an anti-NAT10 antibody. (B) The indicated plasmids were coexpressed in HCT116 cells. Cell lysates were prepared and subjected to immunoprecipitation using an anti-acetyl-lysine antibody. The acetylation levels of NAT10 were evaluated by Western blot using an anti-NAT10 antibody. (C) Purified His-NAT10 was preacetylated and incubated with purified Flag-Sirt1 in the absence or presence of 1 mM NAD+. The acetylation levels of NAT10 were evaluated by Western blot with an anti-acetyl-lysine antibody. (D) Flag-Sirt1 was transfected into HCT116 cells. Immunoprecipitation was performed with an anti-Flag antibody, and NAT10 expression in the precipitants was determined by Western blot. (E) Flag-NAT10 was transfected into HCT116 cells. Immunoprecipitation was performed with an anti-Flag antibody, and Sirt1 expression in the precipitants was determined by Western blot. (F) A GST pull-down experiment was performed with GST-Sirt1 and purified Flag-NAT10. The binding of GST-Sirt1 to Flag-NAT10 was detected by immunoblotting with an anti-Flag antibody. (G) A GST pull-down experiment was performed using purified GST-NAT10 deletion mutants and purified Flag-Sirt1. The schematic diagram represents the GST-NAT10 deletion mutant constructs (lower panel). (H) HCT116 cells were cultured in medium containing 1.0 g/l, 0.5 g/l, or 0 g/l glucose for 18 h. The cells were harvested, and immunoprecipitation was performed using an anti-acetyl-lysine antibody. The acetylation levels of NAT10 were evaluated by Western blot using an anti-NAT10 antibody. (I) HCT116 cells were cultured in medium containing 1.0 g/l, 0.5 g/l, or 0 g/l glucose for 18 h. Immunoprecipitation was performed with an anti-Sirt1 antibody, and NAT10 expression in the precipitants was evaluated by immunoblotting. (J) HCT116 cells were transfected with the indicated siRNAs. Cells were cultured in glucose-rich or glucose-free medium for 18 h, harvested and then subjected to immunoprecipitation with an anti-acetyl-lysine antibody. The acetylation levels of NAT10 were evaluated by Western blot using an anti-NAT10 antibody on the immunoprecipitants. See also Supplementary Figure S1.