Abstract
The effective dehydration of glucose to 5‐hydroxymethylfurfural (HMF) has attracted increasing attention. Herein, a series of sulfonic‐acid‐functionalized ionic liquid (IL)–heteropolyacid (HPA) hybrid catalysts are proposed for the conversion of glucose to HMF. A maximum total yield of HMF and levoglucosan (LGA; ≈71 %) was achieved in the presence of pyrazine IL‐HPA hybrid catalyst [PzS]H2PW in THF/H2O–NaCl (v/v 5:1). The mechanism of glucose dehydration was studied by tailoring the Brønsted/Lewis acid sites of the hybrid catalysts and altering the solvent composition. It was found that water and heteropolyanions have a significant effect on the reaction kinetics. Heteropolyanions are able to stabilize the intermediates and promote the direct dehydration of glucose and intermediate LGA to HMF. A small amount of water could facilitate the conversion of glucose to LGA and suppress the dehydration of LGA to levoglucosenone. In addition, the synergetic effect of Brønsted/Lewis acid sites and a little water was conducive to accelerated proton transfer, which improved the yield of HMF from glucose dehydration.
Keywords: biomass, glucose, heteropolyacids, ionic liquids, proton transport
1. Introduction
With the depletion of fossil fuel resources and the increased environmental pollution that results from the use of fossil fuel resources, the exploration and exploitation of renewable resources has become a hot topic in recent years.1 Biomass is being considered as a substitute for fossil fuel to produce sustainable fuel and chemicals.2 In this context, the conversion of cellullosic biomass to HMF has attracted growing interest from both industry and academia.3 As a potentially important platform chemical, HMF can be used to produce a great variety of fine chemicals, liquid fuels, and monomers for polymeric materials.4
A great deal of research work has been contributed to the conversion of cellullosic biomass to HMF in recent years.3 Brønsted acid and Lewis acid/Brønsted acid bifunctional catalysts have been reported for the transformation of biomass to HMF.5 Due to their unique tunable acidity, high proton mobility, and good stability, heteropolyacids (HPAs) are attracting more and more interest.6 HMF yields as high as 70 to 80 % and a selectivity of at least 90 % have been achieved with metal or organic HPA salts (Brønsted/Lewis acid bifunctional catalysts) in fructose dehydration.7 Compared with fructose, however, glucose is a more promising feedstock for the production of HMF owing to its abundance and cheapness. Unfortunately, very low HMF yields are usually obtained when glucose is used.7
As noted by researchers, glucose can take part in various reactions, such as polymerization, isomerization, dehydration, and decomposition in the presence of Brønsted/Lewis acid catalysts (Scheme 1).8 In one known pathway, glucose is first isomerized to fructose in the presence of a Lewis acid catalyst, then the fructose loses three molecules of water to form HMF with the aid of a Brønsted acid.9 Another reaction pathway that has been proved is the direct dehydration of glucose to HMF in the presence of a strong Brønsted acid, but this route is usually more difficult than the former because of the different conformer distributions of glucose and fructose in solution.10 In addition, the formation of LGA and LGO have been observed during Brønsted acid‐catalyzed glucose conversion to HMF.8b This complex network is probably the reason for the low yields of HMF obtained with glucose as the substrate. Therefore, it is of great significance to tailor the strength and the amount of Lewis and Brønsted acid sites to regulate the distribution of intermediates and enhance the selectivity for HMF. For example, Wang et al. found that a choline chloride‐HPA hybrid catalyst, ChH4AlW12O40, gave a 74.8 % yield of LA for cellulose conversion in a mixed H2O/methyl isobutyl ketone (MIBK) solvent.11 Later, they reported another catalyst (ChH2PW12O40) with a modulated structure and acidity. Under catalysis with ChH2PW12O40, the major product was HMF with a yield of 80.7 %.12 Various metal salt acid catalysts for the conversion of maple wood to HMF have been examined by Cai et al., who revealed that the interplay between Brønsted and Lewis acidic groups could promote the formation of HMF.13
Scheme 1.
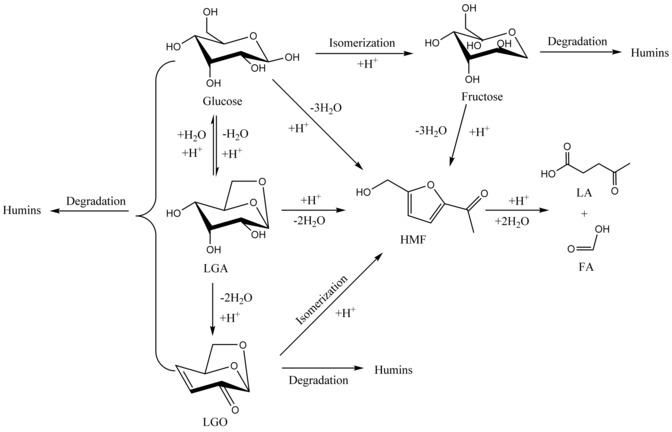
Possible reaction pathways for the dehydration of glucose to HMF (LGA=levoglucosan, LGO=levoglucosenone, LA=levulinic acid, FA=formic acid).8
Apart from the synergistic effect of Lewis and Brønsted acid sites in the catalyst, the solvent plays an important role in the conversion of glucose. Generally speaking, water is the preferred solvent, but pure water did not usually give HMF from glucose in high yields due to the slow rate of HMF formation and rapid rehydration.14 To improve the conversion of glucose to HMF, an effective strategy is to use a biphasic solvent, such as H2O–NaCl and an organic solvent, as the reaction medium.15 In this case, the organic layer acts as an extracting phase to limit the side reaction of HMF and thus improve the HMF yield. Moreover, the nature of the organic solvent also plays a crucial role in regulating the distribution of glucose dehydration intermediates. Huber et al. investigated the effect of mixed solvents that contained a polar aprotic solvent on glucose dehydration.16, 17 In these mixed solvents, apart from HMF, an important chemical precursor (LGO) was also observed in the glucose dehydration reaction. Unfortunately, only single Brønsted acid catalysts, such as H2SO4 and H3PO4, were examined in the mixed solvents. Recently, we studied glucose dehydration with a Brønsted/Lewis bifunctional catalyst in mixed solvents.18 A new, distinct reaction pathway involving the LGO intermediate was proposed. However, the synergistic effect of the Lewis and Brønsted acid groups on the intermediates and products has not been clarified.
In addition, water is an extremely important proton‐transfer bridge in a large majority of charge‐transfer reactions. For example, Iftimie's group studied the proton‐transfer mechanisms by using ab initio molecular dynamics and verified the formation of sequential proton transfer between water and the reactants via encounter complexes.19 It should be noted that glucose dehydration is a typical acid‐catalyzed proton‐transfer reaction. To understand the glucose dehydration mechanism more deeply, therefore, the effect of water in proton transfer must be taken into account.
On the basis of the amino‐functionalized imidazole HPA‐based ionic hybrids that were reported previously,18 herein we prepared a series of novel sulfonic‐acid‐functionalized pyrazine HPA‐based ionic hybrid catalysts, abbreviated as [PzS]nH3−nPW (Scheme 2), which can effectively catalyze glucose dehydration to HMF in THF/H2O–NaCl. The effects of the solvents and the Lewis/Brønsted acid sites of the HPA catalysts on the synthesis of HMF from glucose were investigated. Special attention was given to the roles of the heteropolyanions and the water content on the reaction pathway of HMF formation and the proton‐transfer effect. In addition, the reaction kinetics was studied to understand the reaction mechanism of glucose dehydration catalyzed by HPA‐based ionic hybrid catalysts.
Scheme 2.
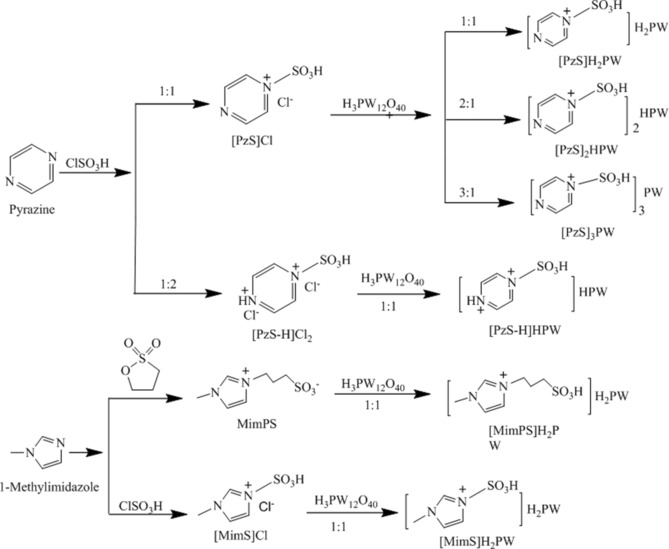
The synthesis of sulfonic‐acid‐functionalized HPA‐based ionic hybrid catalysts.
2. Results and Discussion
2.1. Characterization of Catalysts
The elemental analysis of [PzS]H2PW gave values of 1.61, 0.98, 0.23, and 1.10 wt % for C, N, H, and S, respectively, which is very close to the calculated values (C 1.58, N 0.92, H 0.23, and S 1.05 wt %) according to the suggested chemical composition of [PzS]H2PW in Scheme 2. The SEM‐EDX analysis of C, N, O, P, and W was determined on an extended area of a solid sample of [PzS]H2PW (Figure S1 in the Supporting Information). The weight ratio of N and P is approximately 1:1, and the elemental mapping images disclosed homogeneous dispersions of N, O, S, P, and W across the image area (Figure 1). This evidence confirmed the stoichiometric composition of [PzS]H2PW, which is made up of one sulfonic‐acid‐functionalized pyrazine ionic liquid (IL) cation ([PzS]+) and a HPA anion (H2PW−).
Figure 1.
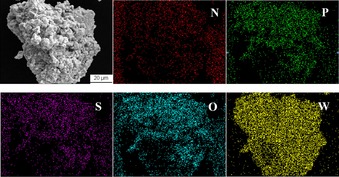
The elemental mapping of [PzS]H2PW.
Figure 2 illustrates the FTIR spectra of H3PW12O40, [PzS]H2PW and [PzS]Cl. For the H3PW12O40 sample, four vibration bands at 1080, 986, 890, and 779 cm−1 (Figure 2, curve a) are characteristic of a Keggin structure for the PW12O40 3− anion, and are attributed to the stretching vibrations of the central oxygen ν(P−Oa), the terminal oxygen ν(W=Ot), the inter‐octahedral oxygen ν(W‐Ob‐W), and the intra‐octahedral oxygen ν(W‐Oc‐W), respectively.20 For the [PzS]H2PW sample (Figure 2, curve b), four Keggin‐featured bands were also observed, which suggested that the Keggin framework was retained even after the HPA proton was substituted by an IL cation [PzS]+. Additionally, characteristic vibration bands for [PzS]Cl (Figure 2, curve c) were detected at 1486, 1174, 614, and 574 cm−1 and were assigned to the stretching vibrations of the pyrazine ring and sulfonic acid group.21 These peaks could be still observed for [PzS]H2PW, which indicated the formation of a sulfonic‐acid‐functionalized pyrazine ionic hybrid through strong ionic‐bond interactions.
Figure 2.
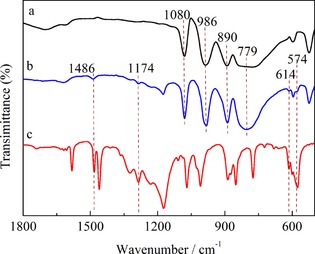
FTIR spectra of a) H3PW, b) [PzS]H2PW, and c) [PzS]Cl.
The acidity of the catalysts was measured by using a Hammett acidity analysis;22 Table 1 summarizes the H 0 values. It can be seen that the acidity decreased in the order H3PW12O40>[PzS]H2PW>[PzS‐H]HPW>[PzS]2HPW>[PzS]3PW>[PzS‐H]Cl2>[PzS]Cl, which is consistent with the values from theoretical calculations according to the chemical formula (see the Supporting Information).
Table 1.
Effect of Lewis/Brønsted acid sites on glucose dehydration.[a]
| Entry | Catalyst | Structure | B/L[b] | H 0 | t [c] | Yield [%] | ||
|---|---|---|---|---|---|---|---|---|
| [mmol g−1] | [h] | LGO | LGA | HMF | ||||
| 1 | none | – | – | – | 6 | 0 | 0 | 0 |
| 2 | pyrazine |

|
0:2 | – | 6 | 0 | 0 | 0 |
| 3 | H3PW12O40 | – | 3:1 | 0.70 | 4 | 0 | 0 | 40.8 |
| 4 | [PzS]Cl |
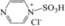
|
1:1 | 0.85 | 3 | 2.3 | 10.4 | 38.7 |
| 5 | [PzS‐H]Cl2 |
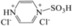
|
2:0 | 0.85 | 2 | 7.0 | 18.2 | 32.9 |
| 6 | [PzS]H2PW |
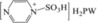
|
3:2 | 0.74 | 6 | trace | 12.8 | 58.2 |
| 7 | [PzS‐H]HPW |
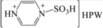
|
3:1 | 0.78 | 5 | trace | 17.8 | 46.4 |
| 8 | [PzS]2HPW |
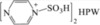
|
3:3 | 0.79 | 6 | trace | 10.2 | 36.1 |
| 9 | [PzS]3PW |
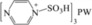
|
3:4 | 0.83 | 6 | trace | 6.4 | 34.3 |
| 10 | [MimS]H2PW |
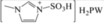
|
– | 0.73 | 5 | 0 | 3.2 | 23.3 |
| 11 | [MimPS]H2PW |
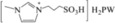
|
– | 0.73 | 5 | 0 | 2.1 | 23.6 |
[a] Reaction conditions: glucose (0.1 g), NaCl (0.37 g), and catalyst (65 μmol) in H2O/THF (12 mL, v/v 1:5) at 180 °C. [b] From the chemical formula, based on the possible number of Lewis or Brønsted acid sites (see the Supporting Information). [c] The reaction time for the maximum yield of HMF.
2.2. The Effect of Lewis/Brønsted Acid Sites on Glucose Dehydration
It is known that the synergistic catalytic effect between Lewis and Brønsted acid sites in a catalyst is beneficial for the dehydration of glucose to HMF. Table 1 shows the effects of Lewis and Brønsted acid sites in sulfonic‐acid‐functionalized HPA‐based ionic hybrids on glucose dehydration at 180 °C in H2O–NaCl/THF (v/v 1:5).
In the absence of acid (Table 1, entry 1) or pyrazine (Table 1, entry 2) as the catalyst, negligible glucose conversion occurred and no detectable products were found after 6 h, which indicated that acidic sites are necessary for the glucose dehydration reaction. H3PW12O40 gave a 40.8 % yield of HMF (Table 1, entry 3), but no LGO or LGA were detected. Additionally, fructose, which is thought to be a common intermediate in the dehydration of glucose to HMF, was not detected. Therefore, it could be concluded that H3PW12O40 is an effective catalyst for the direct conversion of glucose to HMF in H2O–NaCl/THF because, as a strong Brønsted acid, it can donate hydrogen protons for glucose dehydration.23
The sulfonic‐acid‐functionalized pyrazine IL [PzS]Cl, with a 1:1 ratio of Brønsted and Lewis acid sites, gave a 10.4 % yield of LGA, a 2.3 % yield of LGO, and a 38.7 % yield of HMF (Table 1, entry 4), which indicated that [PzS]Cl, similarly to H3PW12O40, has a proton‐donating ability. In contrast to H3PW12O40, glucose conversion to LGA was more conducive than direct dehydration to HMF in the presence of the Brønsted acid sites of the sulfonic acid group. [PzS‐H]Cl2, which has overwhelmingly Brønsted acid sites, gave yields as high as 18.2 (LGA), 32.9 (HMF), and 7 % (LGO; Table 1, entry 5). This suggests that Brønsted acid sites facilitate the conversion of glucose to LGA and the dehydration of LGA to LGO. Additionally, with the aid of strong Brønsted acid sites, glucose also probably underwent various acid‐catalyzed side reactions to give undesired products, such as humins, which as a result decreased the yield of HMF.24 Therefore, we conclude that the species and strength of the acid sites in the catalyst have a significant effect on the distribution of intermediates and the selectivity of the products.
The synthesized HPA‐based ionic hybrid catalyst [PzS]H2PW gave yields of 58.9 % HMF and 12.8 % LGA and trace LGO (Table 1, entry 6). Compared with the yields of [PzS]Cl (Table 1, entry 4), the introduction of a heteropolyanion could accelerate the direct conversion of glucose and LGA to HMF and inhibit LGA dehydration to LGO, which might result from the stabilizing effect of heteropolyanions on the intermediates.25
Furthermore, with a decrease in the proportion of Lewis acid sites in [PzS‐H]HPW, the HMF yield decreased to 46.4 %, whereas the LGA yield increased to 17.8 % (Table 1, entry 7). This suggested that the Lewis acid sites in the catalyst might inhibit the direct dehydration of LGA to HMF. However, with an increase in the proportion of Lewis acid sites in [PzS]2HPW and [PzS]3PW, the HMF yields declined remarkably to 36.1 and 34.4 % accordingly (Table 1, entries 8, 9), which indicated that the synergistic effect of the Brønsted and Lewis acid sites is crucial to the HMF selectivity. Therefore, for the effective conversion of glucose to HMF, the ratio of Lewis and Brønsted acid sites must be optimized to fulfill the synergistic catalytic effect to the utmost extent.
Other two control catalysts, [MimS]H2PW and [MimPS]H2PW, gave yields of 23.3 and 23.6 % HMF, respectively (Table 1, entries 10, 11), and a small amount of LA was detected as a byproduct, which was not detected in the presence of pyrazine ionic hybrids in our experiment. In theory, HMF could be converted into LA in the presence of a strong Brønsted acid. For example, Wang's group investigated the activity of ChH4AlW12O40 for cellulose conversion and obtained a 74.8 % yield of LA in MIBK/H2O–NaCl.12 Although the reason for the formation of LA over [MimS]H2PW and [MimPS]H2PW remains unclear, it might be related to the increase in Brønsted acid sites due to the acidic proton at the C2 position of the imidazolium cation in the catalysts.26 This reconfirmed that the species and strength of the acid have an important influence on the distribution of intermediates and the selectivity of products.
To get a deeper insight into the catalytic mechanism of HPA‐based ionic hybrid catalysts in the H2O–NaCl/THF solvent system, the kinetic behavior of glucose dehydration to HMF by four catalysts was surveyed (Figure 3). The initial rate of formation of HMF decreased in the order [PzS‐H]Cl2>[PzS]Cl>[PzS]H2PW>[PzS‐H]HPW. Under catalysis with [PzS‐H]Cl2 or [PzS]Cl, the initial rate of formation of HMF was in agreement with the proton‐donating ability of the catalyst.16
Figure 3.
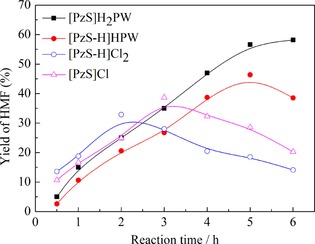
Kinetic behavior of glucose dehydration with different catalysts. Reaction conditions: glucose (0.1 g), NaCl (0.37 g), and catalyst (65 μmol) in H2O/THF (12 mL, v/v 1:5) at 180 °C.
Despite the relatively low initial formation rates of HMF, much higher yields of HMF could be obtained with HPA‐based ionic hybrids [PzS]H2PW and [PzS‐H]HPW. This can be explained by the fact that the heteropolyanions of the ionic hybrid catalyst not only stabilize the reaction intermediates and transition states through effective hydrogen bonding, but also promote the dehydration of glucose and LGA to HMF. Furthermore, the initial formation rate of HMF over [PzS]H2PW was higher than that over [PzS‐H]HPW, which suggested that an appropriate ratio of Brønsted and Lewis acid sites in the catalyst was more favorable for the dehydration of glucose to HMF.
2.3. Solvent Effect on Glucose Dehydration
Polar aprotic solvents have been experimentally proven to be effective in affecting the reaction pathways of biomass dehydration to HMF.27 Different solvents, particularly polar aprotic solvents, were thus evaluated to examine their effect on the glucose dehydration reaction in the presence of [PzS]H2PW at 180 °C; Table 2 presents the product yields. LGA was not detected and very low yields of HMF (5–7 %) were obtained in pure water (Table 2, entry 1) and pure n‐butyl alcohol (nBuOH; Table 2, entry 2). In DMSO (Table 2, entry 3), a yield of HMF as high as 75.6 % was obtained along with trace LGA, which might be related to the high polarity, excellent ability to solubilize glucose, and the effective co‐catalysis effect of DMSO23 compared with the other polar aprotic solvents employed herein. Unfortunately, DMSO is not an ideal solvent due to its high boiling point, which makes the separation and purification of products difficult.
Table 2.
Effect of the solvent on glucose dehydration.[a]
| Entry | Solvent | t [c] | Yield [%] | |||
|---|---|---|---|---|---|---|
| [h] | LGO | LGA | HMF | Total[d] | ||
| 1 | H2O | 6 | 0 | 0 | 6.9 | 6.9 |
| 2 | BuOH | 6 | 0 | 0 | 5.2 | 5.2 |
| 3 | DMSO | 5 | 0 | trace | 75.6 | 75.6 |
| 4 | THF | 6 | 6.7 | 3.4 | 8.5 | 18.6 |
| 5 | dioxane | 6 | 6.4 | 1.2 | 7.8 | 15.4 |
| 6 | GVL | 5 | 4.5 | 1.0 | 6.3 | 11.8 |
| 7 | MIBK | 5 | 5.2 | 3.7 | 6.8 | 15.7 |
| 8 | THF/H2O[b] | 6 | trace | 12.8 | 58.2 | 71.0 |
| 9 | dioxane/H2O[b] | 6 | trace | 8.6 | 37.5 | 46.1 |
| 10 | GVL/H2O[b] | 4.5 | trace | 11.4 | 44.5 | 55.9 |
| 11 | MIBK/H2O[b] | 5 | trace | 2.3 | 16.4 | 18.7 |
| 12 | H2O‐NaCl | 6 | 0 | 0 | 7.5 | 7.5 |
| 13 | THF/H2O | 6 | trace | 2.4 | 20.8 | 23.2 |
[a] Reaction conditions: glucose (0.1 g), [PzS]H2PW (65 μmol), and solvent (12 mL) at 180 °C. [b] The volume ratio of the polar aprotic solvent to the water was 5:1 and NaCl (0.37 g) was added to water. [c] The reaction time for the maximum yield of HMF. [d] The total yield of LGA, LGO, and HMF.
Other solvents with relatively low polarity, such as THF and 1,4‐dioxane (Table 2, entries 4, 5), could lead to the formation of LGO (6–7 %) with low yields of HMF (7–9 %) and LGA (1–4 %). Based on these facts, we speculated that, in aprotic solvents with low polarity, glucose might initially undergo a dehydration reaction with the help of a strong Brønsted acid site to form LGA, then LGA is subsequently dehydrated to LGO. Gamma‐valerolactone (GVL) and MIBK (Table 2, entries 6, 7) gave LGA (1–4 %), LGO (4–6 %), and HMF (6–7 %) despite a relatively higher dipole moment. Compared with pure H2O or nBuOH (Table 2, entries 1,2), polar aprotic solvents are beneficial to the dehydration of glucose to LGA and LGO, which is consistent with the results of Huber and co‐workers, who used sulfuric acid as the catalyst.9b
The yield of HMF can be effectively enhanced in a mixed solvent system (Table 2, entries 8–11) composed of aqueous NaCl and a polar aprotic solvent, which suggested that aqueous NaCl is essential for glucose dehydration because of the very poor solubility of glucose in most polar aprotic solvents. The addition of NaCl to the mixed solvent may also increase the immiscibility of the aqueous and organic phases.28
To confirm the role of NaCl, aqueous NaCl and a mixture of THF and pure water were surveyed separately (Table 2, entries 12, 13). The yield of HMF in aqueous NaCl was almost the same as that in pure H2O system (Table 2, entry 1). A HMF yield of 20.8 % was obtained in THF/pure water (Table 2, entry 13), which is inferior to that in the THF/H2O–NaCl solvent system. The results indicated that the effect of NaCl was mainly to increase the immiscibility of the aqueous and organic phases. Consequently, HMF can be extracted instantly from the aqueous phase into the organic phase and thus prevented from undergoing side reactions, which increases the yield of HMF. Furthermore, it is known that THF is able to stabilize HMF, co‐catalyze glucose dehydration, and inhibit the further hydration of HMF to LA.24 As a result, the highest HMF yield (58.2 %) was obtained in THF/H2O–NaCl (Table 2, entry 8).
Interestingly, much more LGA, but only trace LGO, was formed in the [PzS]H2PW‐catalyzed system in a biphasic mixture of H2O and polar aprotic solvent (Table 2, entries 8–11). That is, in the biphasic system, LGA is a key intermediate in glucose dehydration. There are two probable pathways for the conversion of LGA to HMF: 1) LGA is converted into HMF in a straightforward manner or 2) LGA is first converted into LGO and then isomerized to HMF. To confirm the inference, the reaction of LGA dehydration was also investigated under the standard conditions (NaCl (0.37 g) and [PzS]H2PW (65 μmol) in H2O/THF (12 mL) at 180 °C). A 28 % yield of HMF with trace LGO was obtained after a reaction time of 8 h. It turns out that, in the biphasic system, the presence of water suppresses the dehydration of LGA to LGO and, as a result, only trace LGO was formed. Additionally, LGA could be directly converted into HMF. At the same time, the direct conversion of glucose to HMF could not be ignored in the biphasic solvent system. In water‐free solvents, in contrast, the absence of water probably inhibits the isomerization of LGO to HMF. For this reason, a considerable amount of LGO was observed in the reaction mixture (Table 2, entries 4–7). Conversely, water is likely to accelerate the formation of LGA from glucose. This is a reasonable explanation for the higher LGA yields in the biphasic solvent system compared with single polar aprotic solvent systems. Given the higher HMF yields in the biphasic system (Table 2, entries 8–11), contributions from two reaction pathways are indicated: one in which glucose is directly dehydrated to HMF and the other in which glucose is first converted to LGA and then LGA is transformed to HMF.
In summary, LGA is the key intermediate in glucose dehydration when using [PzS]H2PW as the catalyst. LGA loses a further two molecules of water to form LGO, followed by slow isomerization to HMF in the water‐free polar aprotic solvent system. In a biphasic mixture of water and polar aprotic solvent, water could restrain the conversion of LGA to LGO but benefit the direct conversion of LGA to HMF. Additionally, there is another non‐negligible pathway for the formation of HMF, that is, the direct dehydration of glucose to HMF when using [PzS]H2PW as the catalyst.
2.4. Role of Water Content in the Reaction Pathway
To elucidate the effect of water content on the conversion of glucose to HMF, the water content was increased gradually from 0:1 to 1:0. The results in Figure 4 show that the yields of HMF and LGA first increased and then decreased as the water content was increased, but the yield of LGO invariably declined.
Figure 4.
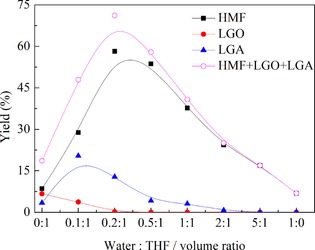
Effect of water on the product distribution from glucose dehydration. Reaction conditions: glucose (0.1 g), NaCl (0.37 g), [PzS]H2PW (65 μmol), and solvent (12 mL) at 180 °C for 6 h.
It is known that water can be tied to proton transfer to facilitate the reaction. In particular, the presence of −SO3H groups in the catalytic system improved the proton‐transfer effect through the formation of encounter complexes, which has been verified by experiments and DFT.19 Therefore, we concluded that a small amount of water in the solvent could form encounter complexes with ‐SO3H groups in the ionic hybrid catalyst through the hydrogen‐bond effect (Scheme 3), and thus facilitate proton transfer between the catalyst and the reactants and increase the HMF yield. As the water content was increased to a certain point, the strong hydrogen‐bond networks between the water molecules became significant and, as a result, the opportunities for encounters between H+ protons and the reactants were decreased and suppressed the formation of HMF, which was confirmed by molecular dynamic (MD) simulations.19
Scheme 3.
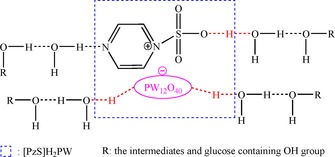
The hydrogen‐bond effect of water molecules and [PzS]H2PW.
Furthermore, the presence of water restrained the conversion of LGA to LGO, which was proved by the declining LGO yield with the increase in water content. Therefore, it can be seen that the addition of a small amount of water to the THF solvent system triggered a dramatic difference in the reaction rate of the tandem reactions and thus led to an increase in the HMF yield, although the detailed effects of the solvent–catalyst–reactants interactions on the reactivity of glucose and LGA needs to be surveyed further.
The reaction kinetics experiments at different temperatures were investigated under the catalysis of [PzS]H2PW in H2O–NaCl/THF (Figure 5). The highest HMF yield was obtained at 180 °C. Although the initial formation rate of HMF was high at 190 °C, a maximum yield of HMF was obtained along with reaction time, which indicated the decomposition of HMF. The formation of humins at high temperature became significant, as revealed by a darkening in the color of the reaction solution. Furthermore, the yield of LGA first increased and then later decreased, but basically remained unchanged as the reaction time was increased. Additionally, only a trace yield of LGO was obtained throughout the dehydration reaction. Again, this indicated that LGA was the intermediate in the glucose dehydration to HMF. The conversion of LGA to LGO was suppressed in the presence of a small amount of water and heteropolyanions. That is, glucose mainly underwent two competitive reaction pathways in our reaction system: direct dehydration of glucose to HMF and dehydration of glucose to LGA followed by further dehydration to HMF.
Figure 5.
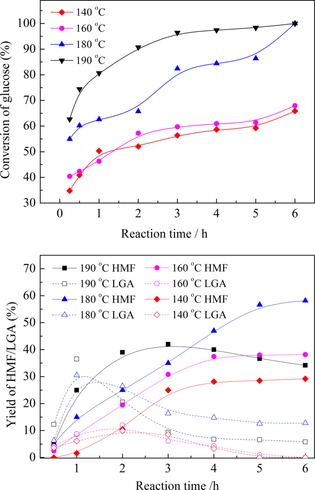
Kinetic behavior of glucose dehydration at different temperatures. Reaction conditions: glucose (0.1 g), NaCl (0.37 g), and catalyst (65 μmol) in H2O/THF (12 mL, v/v 1:5).
3. Conclusions
This study focused on the effects of the solvent and the Brønsted/Lewis acid sites of ionic hybrid catalysts on the dehydration of glucose to HMF. A series of sulfonic‐acid‐functionalized pyrazine HPA‐based ionic hybrid catalysts was prepared and examined for the conversion of glucose to HMF. The maximum total yield of HMF and LGA (71 %) was achieved by using [PzS]H2PW (B/L acid sites 3:2) in THF/H2O–NaCl (v/v 5:1). The introduction of heteropolyanions in the catalyst facilitated the direct conversion of glucose and intermediate LGA to HMF. The synergy of the Brønsted/Lewis acid sites was more favorable for the formation of HMF. Moreover, the effect of the water content in the solvent was systematically surveyed. It was found that a small amount of water could inhibit the dehydration of LGA to LGO and enhance the direct conversion of LGA to HMF. Additionally, the direct dehydration of glucose to HMF was not negligible. The encounter complexes between small amounts of water molecules and the sulfonic acid catalyst were likely to promote proton transfer through effective hydrogen bonding and ionic interactions, and thus improved the selectivity for HMF. This work may be helpful to understand the dehydration of glucose to HMF catalyzed by HPA ionic catalysts and to develop effective HPA catalysts to produce valuable chemicals from biomass.
Experimental Section
Materials and Methods
All chemicals, including glucose, THF, pyrazine, phosphotungstic acid, 1,3‐ propanesultone, and LGA, were purchased from Sinopharm Chemical Reagent Co. and used without further purification. Elemental analyses were performed by using a CHN elemental analyzer (Vario EL cube) and an inductively coupled plasma spectrometer (TCP, Jarrell‐Ash 1100). The 1H NMR spectra were obtained at 400 MHz by using the Hahn echo at RT. The spin rate was 10 kHz. Scanning electron microscopy (SEM; Hitachi S‐4800, accelerating voltage: 5 kV) accompanied by energy‐dispersive X‐ray spectrometry (EDS; accelerating voltage: 20 kV) was used to study the morphology and the elemental distribution. FTIR spectra (KBr discs) were recorded by using a Nicolet 360 FT‐IR instrument in the 4000–400 cm−1 region.
The acidity of the solid samples was measured by using a Hammett acidity analysis according to a literature procedure.22 The Hammett indicator (p‐nitroaniline, 25 mg) and the catalyst (50 mg) were added to deionized water (10 mL and the mixture was stirred at RT for 12 h, then the solid was separated by centrifugal filtration. The solution was measured by using a UV spectrophotometer at λ=380 nm. Each test was done in triplicate and the reported data is the average value.
Catalyst Preparation
The synthesis strategy of sulfonic‐acid‐functionalized HPA‐based ionic hybrid catalysts is illustrated in Scheme 2. The sulfonic‐acid‐functionalized pyrazine IL monomer [PzS]Cl was prepared according to previous reports.21 The main experimentation process is as follows. Chlorosulfonic acid (0.01 mol) and pyrazine (0.01 mol) were added to CH2Cl2 (20 mL) in a 50 mL round‐bottomed flask. The reaction mixture was stirred and heated at reflux for 24 h. After the reaction was complete, the CH2Cl2 was decanted and the solid sample was washed with CH2Cl2 (3×30 mL) and dried to give [PzS]Cl as a white powder. Analogous IL [PzS‐H]Cl2 was prepared in a similar procedure by using a 2:1 molar ratio of chlorosulfonic acid and pyrazine. Analogous IL [MimS]Cl was prepared in a similar procedure by using methylimidazole instead of pyrazine. The characterization data of the synthesized ILs are presented in the Supporting Information.
The sulfonic‐acid‐functionalized imidazole IL MimPS was synthesized according to a literature procedure.20b Methylimidazole (0.1 mol) and 1,3‐propane sulfone (0.1 mol) were added to toluene (20 mL) in a 100 mL round‐bottomed flask. The mixture was stirred for 24 h at 50 °C under a nitrogen atmosphere, then the formed precipitate was filtered and washed with CH2Cl2 (3×30 mL) and dried to give MimPS as a white solid (yield: 96 %).
HPA‐based ionic hybrids were prepared by a reaction between an IL and HPA. Typically, [PzS]Cl (5.0 mmol) and H3PW12O40 (5.0 mmol) were dissolved in deionized water (20 mL). Aqueous H3PW12O40 was added dropwise to the [PzS]Cl solution, and then stirred at RT for 8 h. The formed precipitate was filtered and washed with deionized water, then dried to give [PzS]H2PW. In accordance with the above procedure, [PzS]2HPW and [PzS]3PW were prepared by using the corresponding molar ratio of reactants. Analogous hybrids [PzS‐H]HPW, [MimPS]H2PW, and [MimS]H2PW were prepared by using the corresponding IL precursors. The characterization data for the HPA‐based ionic hybrids are presented in the Supporting Information.
Glucose Dehydration Reaction
The catalytic reaction proceeded in a 100 mL steel autoclave lined with Teflon. In a typical experiment, a mixture of glucose (0.1 g), catalyst (60 μmol), solvent (12 mL), and NaCl (0.37 g) were added to the autoclave, which was then purged three times with nitrogen, heated, and stirred in an oil bath for specified time, then the reaction mixture was cooled to RT immediately. The mixture presented two layers: the upper layer was the organic phase that contained desired products HMF and LGO; the bottom layer was an aqueous phase in which NaCl, traces of HMF, unreacted glucose, the intermediate LGA, and the byproduct humins were dissolved. The qualitative analysis of LGA was carried out by using a gas chromatography‐mass spectrometry instrument (GC‐MS; Perkin–Elmer, Clarus SQ 8) equipped with a capillary column, TC‐1701 (GL Sciences, length 60 m, i.d. 0.25 mm and film thickness 0.25 μm). LGA and glucose were analyzed by using a HPLC instrument (Shimadzu LC‐10A) equipped with a HPX‐87H column and a refractive index detector. Separation was achieved at 30 °C with 5 mm H2SO4 as the mobile phase at a flow rate of 0.6 mL min−1. LGO and HMF were analyzed by using a gas chromatograph (GC6890, Agilent) equipped with a flame ionization detector. The temperature of the oven was kept at 40 °C for 5 min, then heated to 240 °C at a rate of 7.5 °C min−1 and held this temperature for further 15 min. Under optimized conditions, the recycling tests of the water phase in the reaction mixture are described in the Supporting Information. Each test was done in triplicate and the reported data is the average value.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
The authors thank the National Natural Science Foundation of China (nos. 21506118, 21476132, and 51536009), the Provincial Key Research and Development Program of Shandong Province (GG201709210154), and the Natural Science Foundation of Shandong Province (nos. ZR2018MEE011 and ZR2018BB062).
P. Zhao, H. Cui, Y. Zhang, Y. Zhang, Y. Wang, Y. Zhang, Y. Xie, W. Yi, ChemistryOpen 2018, 7, 824.
Contributor Information
Dr. Pingping Zhao, Email: zhaopingping@sdut.edu.cn.
Prof. Hongyou Cui, Email: cuihy@sdut.edu.cn
References
- 1.
- 1a. Wang Y. L., Deng W. P., Wang B. J., Zhang Q. H., Wan X Y., Tang Z. C., Wang Y., Zhu C., Cao Z. X., Wang G. C., Wan H. L., Nat. Commun. 2013, 4, 1–7; [Google Scholar]
- 1b. Zhang Z. R., Song J. L., Han B. X., Chem. Rev. 2017, 117, 6834–6880; [DOI] [PubMed] [Google Scholar]
- 1c. Wang M., Ma J. P., Liu H. F., Luo N. C., Zhao Z. T., Wang F., ACS Catal. 2018, 8, 2129–2165. [Google Scholar]
- 2.
- 2a. Alonso D. M., Bond J. Q., Dumesic J. A., Green Chem. 2010, 12, 1493–1513; [Google Scholar]
- 2b. Mika L. T., Cséfalvay E., Németh Á., Chem. Rev. 2018, 118, 505–613; [DOI] [PubMed] [Google Scholar]
- 2c. Sun Z. H., Fridrich B., Santi A. D., Elangovan S., Barta K., Chem. Rev. 2018, 118, 614–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. van Putten R. J., van der Waal J. C., de Jong E., Rasrendra C. B., Heeres H. J., de Vries J. G., Chem. Rev. 2013, 113, 1499–1597; [DOI] [PubMed] [Google Scholar]
- 3b. Zakrzewska M. E., Bogel-Łukasik E., Bogel-Łukasik R., Chem. Rev. 2011, 111, 397–417; [DOI] [PubMed] [Google Scholar]
- 3c. Qin Y. Z., Zong M. H., Lou W. Y., Li N., ACS Sustainable Chem. Eng. 2016, 4, 4050–4054; [Google Scholar]
- 3d. Wang J., Xi J., Wang Y., Green Chem. 2015, 17, 737–751. [Google Scholar]
- 4.
- 4a. Liu L., Chang H. M., Jameel H., Park J. Y., Park S., Ind. Eng. Chem. Res. 2017, 56, 14447–14453; [Google Scholar]
- 4b. Guo F., Fang Z., Tian X. F., Long Y. D., Jiang L. Q., Bioresour. Technol. 2011, 102, 6469–6472; [DOI] [PubMed] [Google Scholar]
- 4c. Moreau C., Naceur M., Gandini A., Top. Catal. 2004, 27, 11–30; [Google Scholar]
- 4d. Sherwood J., De bruyn M., Constantinou A., Moity L., McElroy C. R., Farmer T. J., Duncan T., Raverty W., Hunt A. J., Clark J. H., Chem. Commun. 2014, 50, 9650–9652. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Su Y., Chang G., Zhang Z., Xing H., Su B., Yang Q., Ren Q., Yang Y., Bao Z., AIChE J. 2016, 62, 4403–4417; [Google Scholar]
- 5b. Nikolla E., Román-Leshkov Y., Moliner M., Davis M. E., ACS Catal. 2011, 1, 408–410; [Google Scholar]
- 5c. Jiménez-Morales I., Moreno-Recio M., Santamaría-González J., Maireles-Torres P., Jiménez-López A., Appl. Catal. B 2015, 164, 70–76. [Google Scholar]
- 6.
- 6a. Deng W. P., Zhang Q. H., Wang Y., Catal. Today 2014, 234, 31–41; [Google Scholar]
- 6b. Deng W., Zhang Q., Wang Y., Dalton Trans. 2012, 41, 9817–9831. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Fan C. Y., Guan H. Y., Zhang H., Wang J. H., Wang S. T., Biomass Bioenergy 2011, 35, 2659–2665; [Google Scholar]
- 7b. Qu Y. S., Huang C. P., Zhang J., Chen B. H., Bioresour. Technol. 2012, 106, 170–172. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. van Zandvoort I., Wang Y., Rasrendra C. B., van Eck E. R., Bruijnincx P. C., Heeres H. J., Weckhuysen B. M., ChemSusChem 2013, 6, 1745–1758; [DOI] [PubMed] [Google Scholar]
- 8b. He J. Y., Liu M. J., Huang K. F., Walker T. W., Maravelias C. T., Dumesic J. A., Huber G. W., Green Chem. 2017, 19, 3642–3653; [Google Scholar]
- 8c. Saha B., Abu-Omar M. M., Green Chem. 2014, 16, 24–38. [Google Scholar]
- 9.
- 9a. Choudhary V., Mushrif S. H., Ho C., Anderko A., Nikolakis V., Marinkovic N. S., Frenkel A. I., Sandler S. I., Vlachos D. G., J. Am. Chem. Soc. 2013, 135, 3997–4006; [DOI] [PubMed] [Google Scholar]
- 9b. Tang J., Guo X., Zhu L., Hu C., ACS Catal. 2015, 5, 5097–5103. [Google Scholar]
- 10.
- 10a. Kuster B., Starch/Staerke 1990, 42, 314–321; [Google Scholar]
- 10b. Zhu Y., Zajicek J., Serianni A. S., J. Org. Chem. 2001, 66, 6244–6251; [DOI] [PubMed] [Google Scholar]
- 10c. Lin H., Xiong Q., Zhao Y., Chen J., Wang S., AIChE J. 2017, 63, 257–265. [Google Scholar]
- 11. Sun Z., Xue L. F., Wang S. T., Wang X. H., Shi J. Y., Green Chem. 2016, 18, 742–752. [Google Scholar]
- 12. Zhang X. Y., Zhang D., Sun Z., Xue L. F., Wang X. H., Jiang Z. J., Appl. Catal. B 2016, 196, 50–56. [Google Scholar]
- 13.
- 13a. Cai C. M., Nagane N., Kumar R., Wyman C. E., Green Chem. 2014, 16, 3819–3829; [Google Scholar]
- 13b. Cai C. M., Zhang T., Kumar R., Wyman C. E., Green Chem. 2013, 15, 3140–3145. [Google Scholar]
- 14. Mukherjee A., Dumont M. J., Raghavan V., Biomass Bioenergy 2015, 72, 143–183. [Google Scholar]
- 15. Pagan-Torres Y. J., Wang T., Gallo J. M. R., Shanks B. H., Dumesic J. A., ACS Catal. 2012, 2, 930–934. [Google Scholar]
- 16. Cao F., Schwartz T. J., McClelland D. J., Krishna S. H., Dumesic J. A., Huber G. W., Energy Environ. Sci. 2015, 8, 1808–1815. [Google Scholar]
- 17. Walker T. W., Chew A. K., Li H. X., Demir B., Zhang Z. C., Huber G. W., Van Lehn R. C., Dumesic J. A., Energy Environ. Sci. 2018, 11, 617–628. [Google Scholar]
- 18. Zhao P. P., Zhang Y. Y., Wang Y., Cui H. Y., Song F., Sun X. Y., Zhang L. P., Green Chem. 2018, 20, 1551–1559. [Google Scholar]
- 19.
- 19a. Thomas V., Rivard U., Maurer P., Bruhács A., Siwick B. J., Iftimie R., J. Phys. Chem. Lett. 2012, 3, 2633–2637; [DOI] [PubMed] [Google Scholar]
- 19b. Sagarik K., Phonyiem M., Lao-ngam C., Chaiwongwattana S., Phys. Chem. Chem. Phys. 2008, 10, 2098–2112. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Misono M., Catal. Rev. Sci. Eng. 1987, 29, 269; [Google Scholar]
- 20b. Leng Y., Wang J., Zhu D., Ren X., Ge H., Shen L., Angew. Chem. Int. Ed. 2009, 48, 168–171; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 174–177. [Google Scholar]
- 21. Li J., Li D. F., Xie J. Y., Liu Y. Q., Guo Z. J., Wang Q., Lyu Y., Zhou Y., Wang J., J. Catal. 2016, 339, 123–134. [Google Scholar]
- 22. Moosavi-Zare A. R., Zolfigol M. A., Khakyzadeh V., Böttcher C., Beyzavi M. H., Zare A., Hasaninejad A., Luque R., J. Mater. Chem. A 2014, 2, 770–777. [Google Scholar]
- 23.
- 23a. Amarasekara A. S., Williams L. D., Ebede C. C., Carbohydr. Res. 2008, 343, 3021–3024; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23b. Wang S. S., Yang G. Y., Chem. Rev. 2015, 115, 4893–4962. [DOI] [PubMed] [Google Scholar]
- 24.
- 24a. Li Z. H., Su K. M., Ren J., Yang D. J., Cheng B. W., Kim C. K., Yao X. D., Green Chem. 2018, 20, 863–872; [Google Scholar]
- 24b. Zhao Y., Wang S. R., Lin H. Z., Chen J. P., Xu H., RSC Adv. 2018, 8, 7235–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.
- 25a. Wang S., Lin H., Chen J., Zhao Y., Ru B., Qiu K., Zhou J., RSC Adv. 2015, 5, 84014–84021; [Google Scholar]
- 25b. Li X. C., Peng K. H., Liu X. H., Xia Q. N., Wang Y. Q., ChemCatChem 2017, 9, 2739–2746. [Google Scholar]
- 26. Chidambaram M., Bell A. T., Green Chem. 2010, 12, 1253–1262. [Google Scholar]
- 27.
- 27a. Wang T., Nolte M. W., Shanks B. H., Green Chem. 2014, 16, 548–572; [Google Scholar]
- 27b. Weingarten R., Beuerman A. R., Cao F., Luterbacher J. S., Alonso D. M., Dumesic J. A., Huber G. W., ChemCatChem 2014, 6, 2229–2234. [Google Scholar]
- 28. Weingarten R., Tompsett G. A., Conner W. C., Huber G. W., J. Catal. 2011, 279, 174–182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


