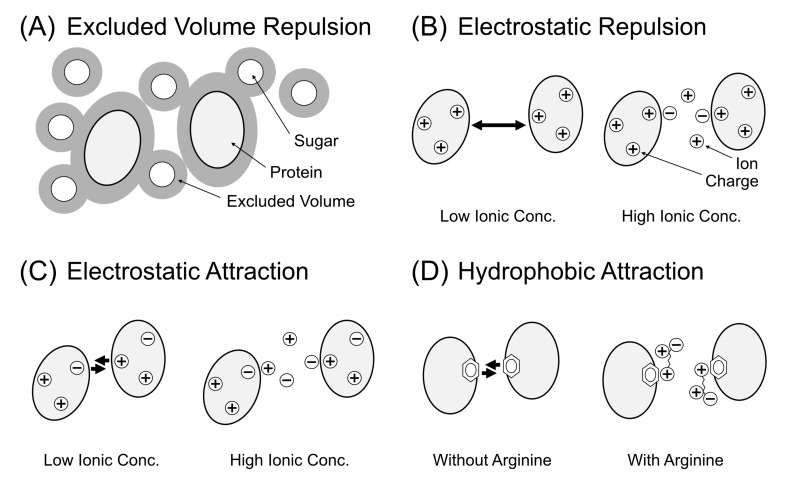
Fig. (1).
Summary of the strategy to decrease the viscosity of protein solution using small molecular additives. (A) The case of exclusion volume effect by steric repulsion. Small solutes (sugar molecules) hamper the mobility of proteins. Thus, the removal of the solutes can decrease the solution viscosity. (B) The case of electrostatic repulsion between proteins. Bold arrows show higher repulsive force between proteins. In the high ionic concentration, the repulsive force decreases, leading to a decrease in the viscosity. (C) The case of electrostatic attraction by charge–charge and charge–dipole interactions. Ions can decrease the viscosity by electrostatic shield by the prevention of the local interactions. (D) The case of hydrophobic interaction between nonpolar and aromatic surfaces of proteins. Arginine molecules weaken the hydrophobic interaction.