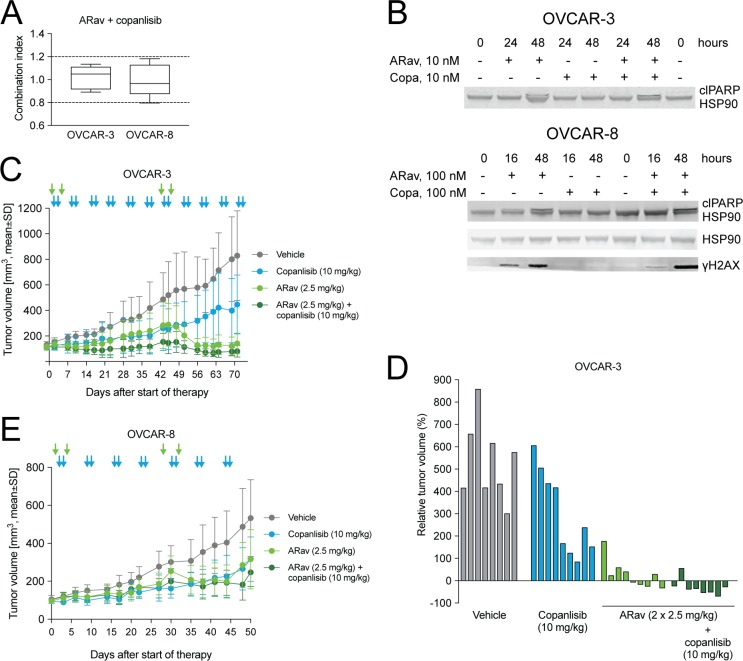
Figure 6. Antitumor efficacy of anetumab ravtansine in combination with copanlisib in preclinical ovarian cancer models in vitro and in vivo.
(A) Combination of anetumab ravtansine with copanlisib in OVCAR-3 and OVCAR-8 cells in vitro. The activity was determined as additive based on the determined combination indices (CI) between 0.8 and 1.2 (n = 5). (B) Cell lysates were analyzed for γH2AX, cleaved PARP1 and HSP90 by Western blot at the indicated time points. (C) Tumor growth in the OVCAR-3 ovarian cancer model (n = 9). Treatments were initiated 43 days after tumor cell inoculation. (D) Changes in the relative volume of OVCAR-3 tumors in panel A on day 71 after start of therapy, represented as a percentage of the initial tumor volume in each individual mouse. (E) Tumor growth in the OVCAR-8 ovarian cancer model (n = 7–9). Treatments were initiated 21 days after tumor inoculation. Anetumab ravtansine (i.v.) and/or copanlisib (i.v.) were administered as indicated by arrows. ARav, anetumab ravtansine; Copa, copanlisib.