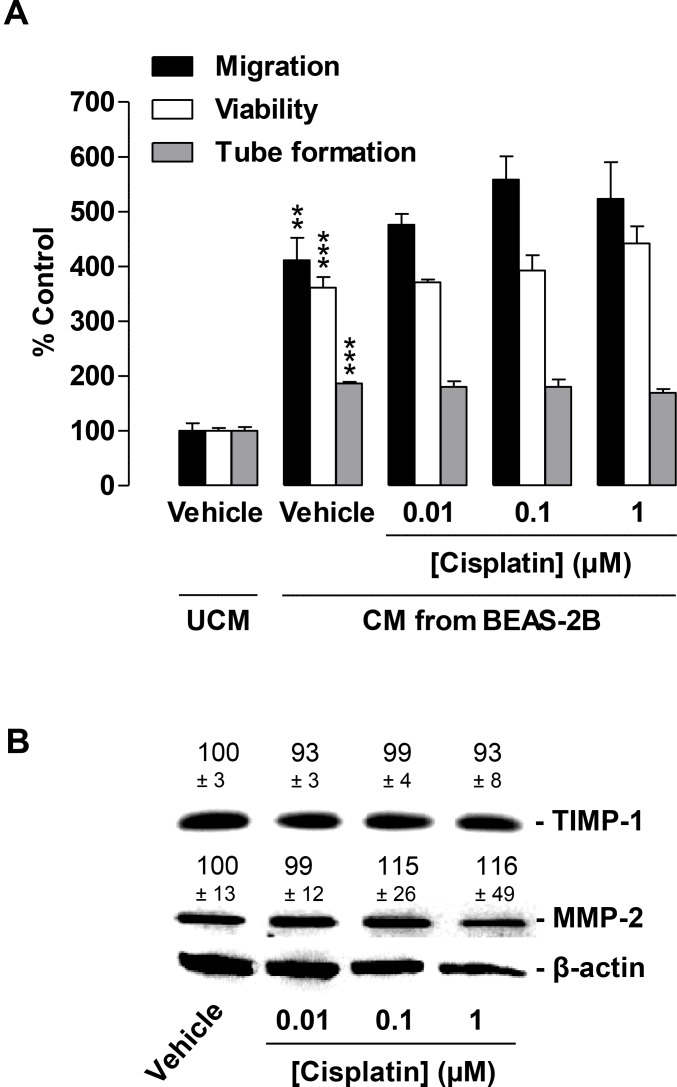
Figure 7. Impact of conditioned media (CM) obtained from cisplatin-treated non-cancer bronchial epithelial BEAS-2B cells on migration, viability, and tube formation of HUVECs.
(A), Migration (black bars, modified Boyden chamber assay), viability (white bars, WST-1 assay) and tube formation (grey bars) of HUVECs following resuspension in serum-free vehicle-containing DMEM (unconditioned media [UCM]) or CM from vehicle- or cisplatin-treated BEAS-2B cells. BEAS-2B cells were incubated for 48 h with vehicle or the indicated concentrations of cisplatin. Incubation time of HUVECs was 24 h in migration and viability assays. Tube formation was measured following a 2-h exposure of HUVECs to CM. Percentage of control represents comparison with UCM (set as 100%), i.e., vehicle-treated HUVECs in serum-free DMEM in the absence of cisplatin and BEAS-2B cells. (B), Western blotting analysis of TIMP-1 and MMP-2 release into CM of BEAS-2B cells treated with vehicle or the indicated concentrations of cisplatin for 48 h. Western blot images are representative of each experiment. Values above the blots are the mean ± SEM and represent alterations in TIMP-1 and MMP-2 protein levels in CM in comparison with vehicle-treated cells (set as 100%), according to densitometric analyses. Western blotting analysis of CM was supplemented with β-actin analysis of the respective cell lysates. Values are the mean ± SEM of n = 3 (A) or n = 6 (B). **p < 0.01; ***p < 0.001 vs. corresponding vehicle control, one-way ANOVA plus a post-hoc Bonferroni test. Statistical analyses did not yield significant differences between cisplatin-treated groups (third, fourth, or fifth triplet) and the corresponding vehicle-treated group (second triplet). For reasons of clarity, statistical differences (all at p < 0.001) between first versus third, fourth, or fifth triplet are not indicated.