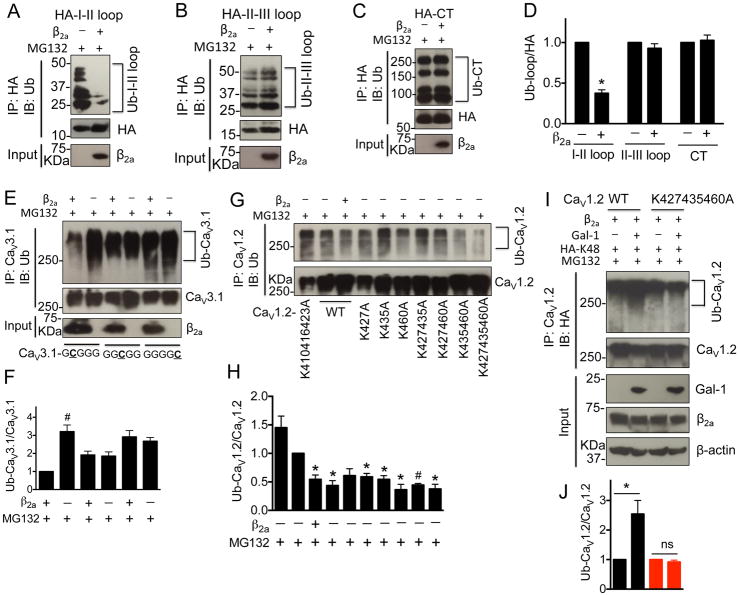
Figure 2. CaVβ subunits prevent the CaV1.2 ubiquitination by masking the lysines (K427, K435 and K460) within I–II loop.
(A–D) Western blots and quantifications of ubiquitinated I–II loop, II–III loop and C-terminus in the presence or absence of β2a subunits in tranfected HEK 293 cells treated with MG132 (1 μM) for 16 h (n=4). (E, F) Western blots and quantifications of ubiquitinated chimeric CaV3.1 channels containing CaV1.2 I–II loop, II–III loop or C-terminus in the presence or absence of β2a subunits in tranfected HEK 293 cells treated with MG132 (1 μM) for 16 h (n=4). (G, H) Western blots and quantifications of ubiquitinated full length CaV1.2 channels with mutations of lysines within I–II loop into alanines in the presence or absence of β2a subunits in tranfected HEK 293 cells treated with MG132 (1 μM) for 16 h (n=5). (I, J) Western blots and quantifications of ubiquitinated CaV1.2-77wt or CaV1.2-K427435460A channels in HEK 293 cells co-transfected with β2a subunit, HA-Ub-K48 (All lysines were mutated except K48) in the presence or absence of Gal-1 (n=4). Cell lysates were harvested after MG132 (1 μM) treatment for 16 h. Data were shown as mean ± SEM. *p<0.05, #p<0.01 versus control group.