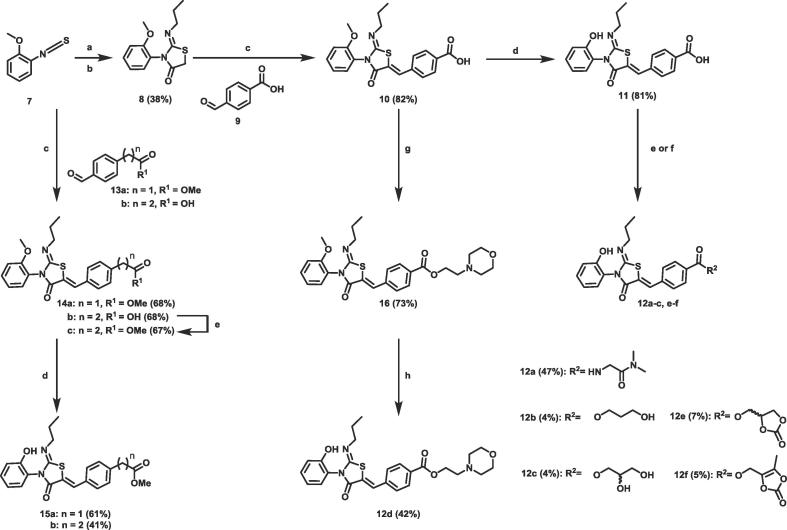
Scheme 2.
Reagents and conditions for preparation of compounds 11, 12a–f and 15a, b: (a) n-PrNH2 (1 eq), CH2Cl2, rt (b) 2-bromoacetyl bromide (1 eq) and pyridine (2 eq), CH2Cl2, 0 °C to rt (two steps) (c) aldehyde (1 eq), NaOAc (2 eq), AcOH, 85 °C (d) BBr3 (8 eq), CH2Cl2, −78 °C to rt (e) For 12a–c. amine (3 eq) or alcohol (5 eq), HOAt (1.5 eq), EDC (1.5 eq), DIPEA (2.5 eq), CH2Cl2, rt (f) For 12d–f. alcohol, alkyl chloride or alkyl tosylate (1.1–1.5 eq) K2CO3 (1.5 eq), NaI (0.1 eq), DMF, 70 °C (g) NEt3 (3 eq), EDC (1.1 eq), HOAt (1.1 eq), alcohol (1.5 eq) rt (h) BBr3 (5 eq), CH2Cl2, −78 °C to rt. eq: equivalent; rt: room temperature; HOAt: 1-Hydroxy-7-azabenzotriazole; EDC: 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide; DIPEA: N,N-Diisopropylethylamine; DMF: dimethyl formamide.