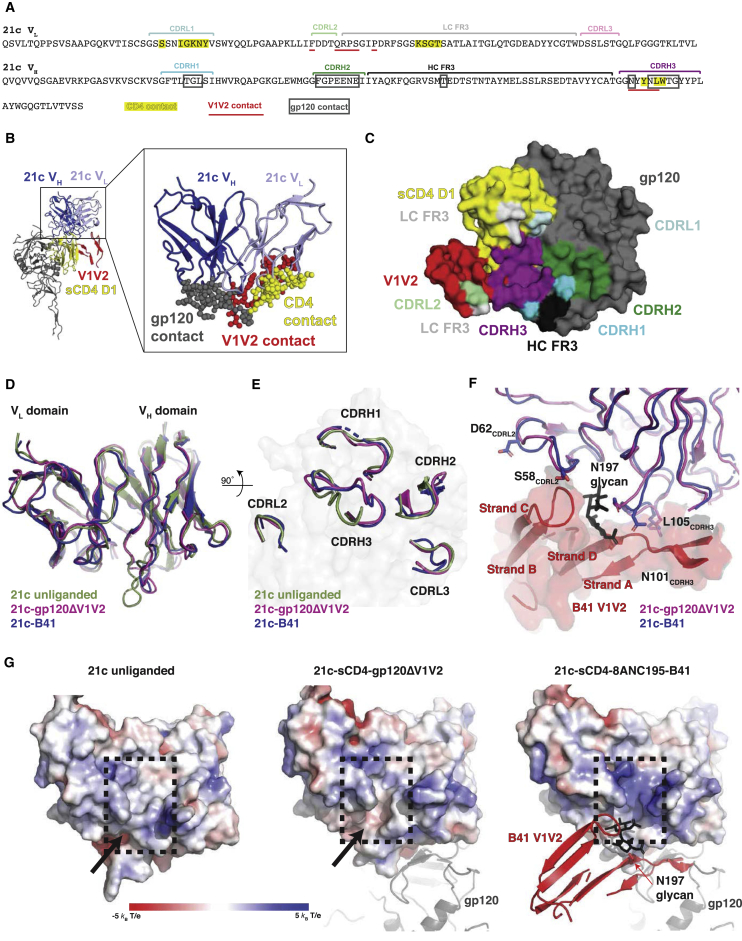
Figure 6.
21c Epitope on gp120 and sCD4
(A) Sequence of 21c VHVL with CDR residues indicated by brackets, and sCD4-contacting residues highlighted in yellow, V1V2-contacting residues in red, and gp120-contacting residues in gray. Contacting residues in the 21c paratope and epitope were defined as two residues containing an atom within 5 Å of each other.
(B) Structure of a 21c, sCD4, gp120 protomer in the B41-sCD4-21c-8ANC195 structure, with inset showing residues contacting V1V2, sCD4, and gp120 highlighted.
(C) 21c epitope on a gp120-sCD4 complex (surface representation seen from the top). Highlighted regions of the sCD4 and gp120 surfaces are contacted by the indicated regions of 21c. To prevent overinterpretation of contacts at low resolution, the displaced V1V2 was modeled as polyalanine unless side-chain density at ≥7σ (0.04 e/Å3) was observed.
(D) Superimposition of VHVL domains from structures of unliganded 21c Fab (PDB: 3LMJ; green), 21c Fab bound to a sCD4 complex with monomeric gp120ΔV1V2 core (PDB: 3LQA; magenta), and 21c from the B41-sCD4-21c-8ANC195 complex (this study; blue).
(E) View of CDRs in the superimposition shown in (D).
(F) Close up of 21c interaction with V1V2 and Asn197gp120 glycan.
(G) Comparison of electrostatic surface potentials (positive potential shown as blue, negative potential shown as red) of unliganded 21c (left), sCD4 plus gp120 core-bound 21c (middle), and B41 Env trimer plus sCD4-bound 21c (right) showing the opening of a positively charged cleft (black arrow and box) that could accommodate negatively charged terminal sialic acids on the complex-type Asn197gp120 glycan on V1V2 (right panel; black sticks).