Fig. 5.
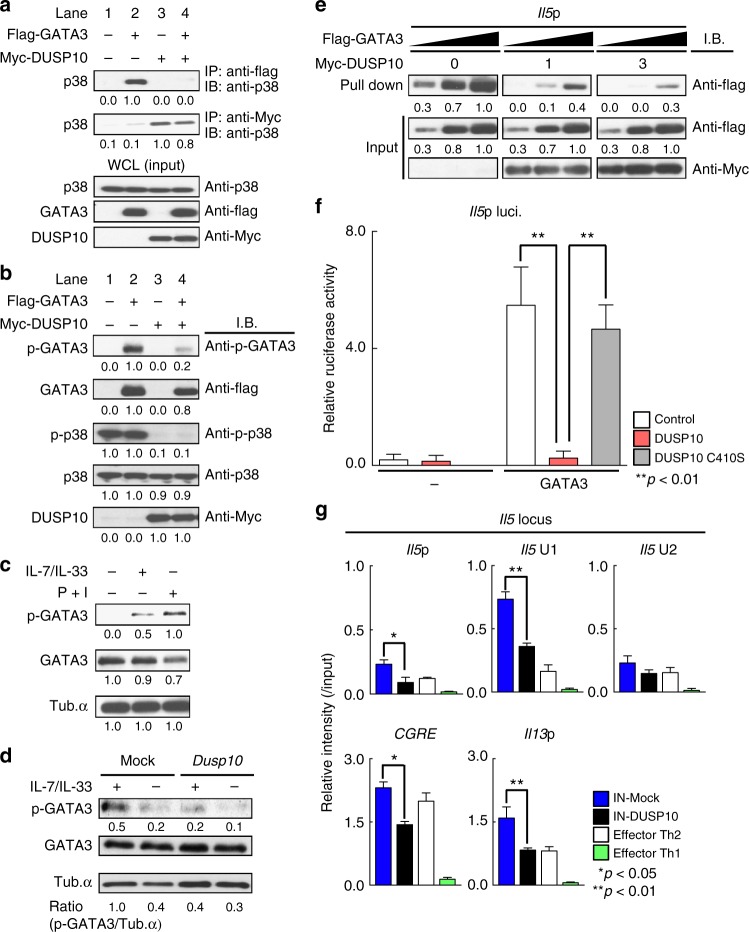
DUSP10 suppresses the transcriptional activity of GATA3. a, b 293T cells were transfected with Myc-tagged DUSP10 or Flag-tagged GATA3. a Immunoprecipitation assay was performed with anti-Myc or anti-Flag. Immunoblotting of whole-cell lysates is also shown as a control (Input). b The phosphorylated GATA3 (p-GATA3), total GATA3 (GATA3), phosphorylated p38 (p-p38), total p38 (p38) and total DUSP10 (DUSP10) in whole fraction. c The phosphorylated GATA3 (p-GATA3) and total GATA3 (GATA3) in the nuclear fraction from Tpath2 cells. Tpath2 cells were stimulated with P + I, or IL-7 + IL-33 for 30 min. d The phosphorylated GATA3 (p-GATA3) and total GATA3 (GATA3) in the nuclear fraction from Dusp10-overexpressing ILC2s shown in Fig. 4b. ILC2s were stimulated with IL-7 + IL-33 for 30 min. The arbitrary ratio of the intensity of p-GATA3 to that of tubulin was shown. e 293 T cells were transfected with Myc-tagged DUSP10 or Flag-tagged GATA3, and total extracts were subjected to a pull-down assay using Il5 promoter oligonucleotide as described in the Supplementary information. Immunoblotting of total cell lysates is also shown (Input). The x-axis of GATA3 refers to the amounts of cell lysates (3-fold doses; DNA-precipitants and input samples) that were blotted with anti-Flag Ab. Band intensities were measured with a densitometer and arbitrary densitometric units are shown (a–e). f Reporter assays with the Il5 promoter were performed using the Dusp10-transfected D10.G4.1 Th2 cell line. The mean values with standard deviations of relative luciferase activity of three different experiments are shown. g A ChIP assay was performed with anti-GATA3 at the Il5 gene locus, CGRE and Il13p in Control- or Dusp10-overexpressing ILC2s as shown in Fig. 4b, and effector Th1 cells and effector Th2 cells. a–g Three independent experiments were performed with similar results (**p < 0.01; *p < 0.05; Mann–Whitney U test; f, g). Three technical replicates were performed for reporter (f) and ChIP (g) assays