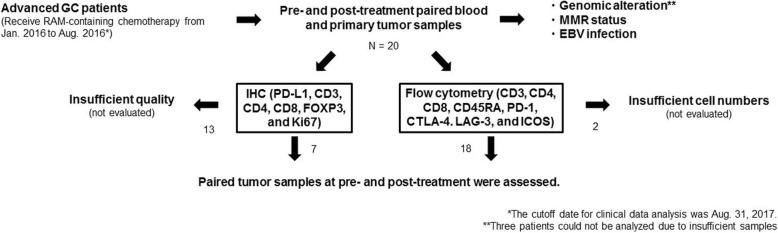
Fig. 1.
The scheme of our study. We prospectively enrolled patients with advanced GC who were scheduled to receive RAM-containing chemotherapy from January 2016 to August 2016. We obtained pre- and post-treatment paired blood and primary tumor samples by endoscopic biopsy to assess immune profiles from 20 patients. Archived FFPE samples were used for analyses of genomic alterations, MMR status, and EBV status. Tumor samples from 7 and 18 patients were subject to IHC and flow cytometry, respectively