Abstract
New discoveries into the functional role of primary cilia are on the rise. In little more than 20 years, research has shown the once vestigial organelle is a signaling powerhouse involved in a vast number of essential cellular processes. In the same decade that interest in primary cilia was burgeoning, nitric oxide won molecule of the year and a Nobel prize for its role as a near ubiquitous signaling molecule. Although primary cilia and nitric oxide are both involved in signaling, a direct relationship has not been investigated; however, after a quick review of the literature, parallels between their functions can be drawn. This review aims to suggest a possible interplay between primary cilia and nitric oxide signaling especially in the areas of vascular tissue homeostasis and cellular proliferation.
Keywords: primary cilia, nitric oxide, signaling
1. Introduction
Understanding the roles of primary cilia in the human body is still in its infancy; however, the last several decades have produced a wealth of new information about the formerly functionless organelle. Cilia can be found in almost every cell of the body, often having a specialized sensory function [1, 2]. When cilia malfunction, severe and multisystemic abnormalities known as ciliopathies occur. The list of ciliopathies is ever expanding as mutations in over 40 genes have been discovered to alter ciliary structure or function with over 1,000 polypeptides in the ciliary proteome yet to be fully investigated [3].
Cilia gained notoriety through their involvement in the pathogenesis of Polycystic Kidney Disease (PKD) as fluid mechanosensors in the kidney. Outside of renal abnormalities, the cardiovascular system is also greatly affected by the disease leading researchers to investigate the role of cilia in the fluid filled vascular system. Nauli et al. postulated that primary cilia activation in vascular endothelial cells would lead to a similar calcium influx as observed in kidney tubule epithelia; the group demonstrated that vascular cilia did play a similar role in sensing fluid shear stress and the corresponding increase in calcium levels correlated with nitric oxide (NO) release, thus contributing to blood pressure control. Evidence from cilia mutant cell lines showing little to no calcium influx and no nitric oxide release when subjected to shear stress was also provided in support of this hypothesis [4–6]. Nitric oxide is a ubiquitous signaling molecule with essential functions in almost every organ system [7–9]. A number of pathologies are associated with aberrant NO production or bioavailability due to abnormal signaling cascades. This will occasionally coincide with abnormal ciliaregulated signaling pathways. With a defined connection between cilia and NO in the vasculature and an overlap between signaling pathways in other pathologies, the obvious question becomes “is there a connection between primary cilia and NO?”However, little research is available on the subject and a direct link between NO-related signaling and cilia dysfunction has yet to be demonstrated [10, 11]. Thus, this review aims to suggest a critical and complex link between cilia and nitric oxide that extends beyond vasodilation.
2. Primary cilia
Primary cilia are non-motile single cellular extensions that can be found on a majority of mammalian cells including but not limited to endothelia, epithelia, and neurons[12–15]. The cytoskeleton of primary cilia, known as the axoneme, consists of 9 concentric doublet microtubules (9+0 [16]. Stemming from the basal body, the cilium is constructed using bi-directional intraflagellar transport (IFT) molecules. The axoneme acts as a scaffold for various protein complexes, such as kinesins and dyneins, that facilitate antero- and retrograde trafficking of cargo proteins along the ciliary shaft [17]. The ciliary membrane is continuous with the cellular membrane; however, it has a distinct composition of receptors and integral proteins due to the ciliary transition zone. The latter is a region between the basal body and the axoneme responsible for the compartmentalization of the cilia while also providing docking sites to enable the transport of molecules in and out of the cilioplasm (Figure 1) [18–20]. There are several proposed mechanisms for the trafficking of molecules to the cilia. Briefly, transmembrane proteins are often associated with a specific protein sequence that targets ciliary localization, one example being the N-terminal RVxP sequence on polycystin-2 [21, 22]. Active transport of vesicles from the golgi apparatus to specific docking sites at the transition zone is another proposed mechanism [23]. Similar to how importins and exportins function at nuclear pores, the vesicles are believed to interact with exocyst complexes and undergo SNARE (Soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor) mediated diffusion across the barrier separating the cilioplasm from the cellular cytoplasm [24]. The BBSome, an octameric protein complex and a component of the basal body and is involved in trafficking transmembrane proteins to the ciliary membrane through a combination of the mechanisms listed above. BBSomes are capable of recognizing ciliary targeting sequences and interacting with several molecules upstream of Rab8 activation. Although they are not required for ciliogenesis, the failure of BBSomes to deliver specific proteins to the cilia can lead to loss of ciliary function [22, 25–27].
Figure 1: Primary cilia structure.
The axoneme of primary cilia is mainly anchored from the basal body and enclosed within the ciliary membrane which is continuous with the plasma membrane. The basal body is composed of the mother and the daughter centrioles and some transition fibers to anchor the basal body to the cell membrane. The ciliary membrane hosts specific membrane and protein receptors that facilitate proper cilia signaling (Left panel). Primary cilia found on the surface of vascular endothelial cells can be identified by a simple immunofluorescence technique utilizing antibody against acetylated α-tubulin (green) to label primary cilia and pericentrin (red) to label centriole or basal body. The nucleus is counterstained with DAPI to label DNA (Right panel). Left panel is adopted from [73].
Primary cilia function as sensory hubs, housing a variety of mechanosensory proteins, chemosensory receptors, and ion channels to translate the extracellular stimuli into an intracellular biochemical signal triggering a cellular response. Due to their extensive sensory role, much research has been dedicated to understanding how cilia organize signaling cascades. Currently, there are two main models: the compartment model and the scaffold model. The compartment model suggests that the ciliary ultrastructure itself is essential for proper signaling whereas the scaffold model suggests that after stimuli, the diffusion of signaling molecules and secondary messengers is not sufficient on its own, thus requiring IFT molecules to scaffold signaling components or import specific transduction intermediates into the cilia (28 or 29). A more detailed explanation of these topics can be found in (28 or29 could be up through 37) as the complex mechanisms of these models are beyond the scope of this review, thus we will only focus on the relevant signaling cascades the lead to NO production. The inferred interplay between primary cilia and nitric oxide will mainly be discussed in the context of vasodilation, wound healing, dopamine signaling, and cellular proliferation.
3. Nitric Oxide
Nitric oxide is a gaseous signaling molecule involved in a variety of cellular pathways contributing to the normal functions in a majority of organ systems [38]. It is a highly reactive and readily diffuses across cellular membranes making it an ideal paracrine signaling molecule. NO is mainly synthesized from L-arginine, oxygen, and NADPH in a redox reaction catalyzed by nitric oxide synthase (NOS) [39]. Of the three NOS isoforms, endothelial NOS (eNOS or NOS3) and neuronal NOS (nNOS or NOS1) are constitutively expressed in cells and are calcium dependent. Cytokine inducible NOS (iNOS or NOS2) is expressed by macrophages and other pro-inflammatory cytokines as needed [11]. iNOS and nNOS are both soluble enzymes existing within the cytosol; whereas eNOS is largely membrane associated, specifically localizing to the plasma or the golgi body membranes. The unique cellular and subcellular distribution of NOS may contribute to its diverse functions throughout the body [40].
4. Cilia and NO interplay
4.1. Vasodilation
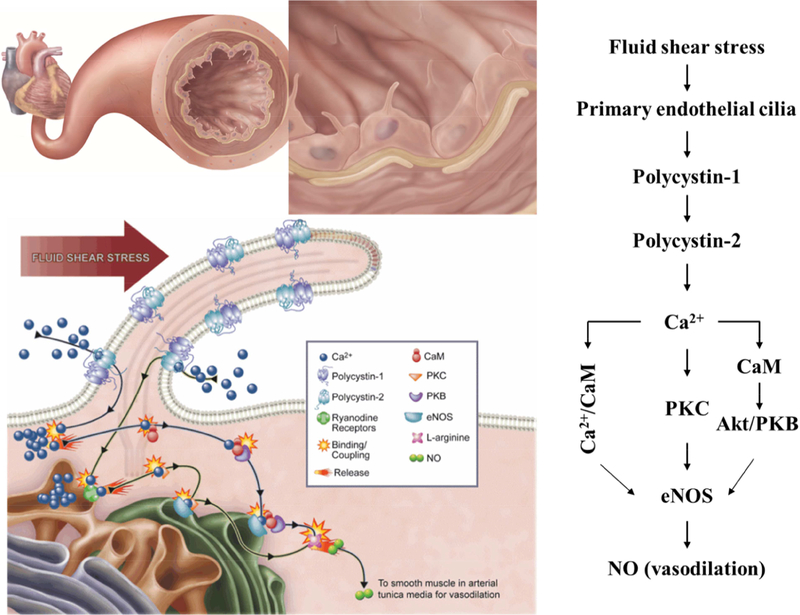
The majority of research on the connection between cilia and NO centers around the vasculature. Both primary cilia and NO have well defined independent roles within the vascular system. However, recent studies have suggested a direct relationship between the two. Vascular endothelial cells line the blood vessel wall and are in continuous contact with fluid shear stress generated by blood flow. It has long been established that endothelial cells are mechanotransducers of shear stress which triggers the biosynthesis of NO, aiding in the regulation of vascular tone as NO readily diffuses into the surrounding vascular smooth muscle cells producing vasorelaxation [41]. Although a number of mechanosensitive and stretch sensitive receptors can be found on the cell membrane, evidence has supported primary cilia as the chief sensor in this pathway. Polycystin-1 (PC-1), a mechanosensory protein found dysfunctional in PKD, has been shown to localize to vascular endothelial primary cilia. In vitro studies investigating the role of PC-1 as a fluid shear mechanosensor, reported that in contrast to wildtype endothelial cells, PC-1 knockout cells did not produce an increase in cytosolic calcium or a corresponding NO flux in response to fluid shear stress. To demonstrate that the calcium and NO signals are induced in response to ciliary PC-1 activation, the authors utilized Tg737orpk/orpk endothelial cells, which lack ciliary ultrastructure but have functional PC-1. Neither calcium nor NO signals could be observed at flow rates up to 50 dyne/cm2 [5]. This suggested that not only is PC-1 responsible for proper cilia mechanosensory function, but that it was the ciliary PC-1, specifically, that elicited NO production. Further studies by the same group showed that polycystin-2 (PC-2), a calcium permeable cation channel which forms a complex with PC-1, was also essential for mechanotransduction. PC-2 knockdown studies reported a reduction in calcium and NO flux under shear stress compared to control cells. This was confirmed in ex vivo studies where endothelial cells isolated from pkd2−/− mice arteries were unable to respond to fluid shear stress [4]. Taken together, these results indicate that both PC-1 and PC-2 are required for proper cilia mechanosensation and further support that the activation of the PC-1/PC-2 complex initiates the signaling cascade necessary for calcium dependent NO biosynthesis. The authors propose that the increase in intracellular calcium is caused by an increase in intra-ciliary calcium, but others have suggested that calcium could be mobilized in both directions between the cilia and the cytosol [42–44]. The increase in intracellular calcium leads to the formation of the calcium/calmodulin complex which can directly activate constitutive NOSs, such as eNOS by binding to its target site on the enzyme [45]. Calcium/calmodulin has also been shown to indirectly activate eNOS through activation of the AKT/PKB pathway which stimulates AMPK, a known activator of eNOS (Figure 2) [46]. Although eNOS activation is predominantly calcium dependent, some studies suggest a calcium independent pathway is also available, most notably through heat shock protein 90 (HSP90) [47, 48]. Interestingly, HSP90 also localizes throughout the ciliary axoneme. HSP90 is a molecular chaperone but may also act as a signal transducer associated with eNOS in several systems, including the vasculature [48, 49]. Although HSP90 activation can increase eNOS activity in the presence of elevated calcium levels, it can also lead to more prominent eNOS activity at low calcium concentrations as HSP90 binds directly to eNOS and increases its affinity for calmodulin [50, 51].
Figure 2. Primary cilia activation by fluid shear stress and NO signaling in vascular endothelia.
The illustration above depicts the bending of primary cilia during fluid shear stress and the consequent biochemical production and release of nitric oxide (NO) which is dependent on the activation of endothelial primary cilia in the vasculature (Left panel). The bending of cilia by fluid-shear stress activates the mechanosensory polycystins complex and initiates biochemical synthesis and the release of NO. This biochemical cascade involves extracellular calcium influx (Ca2+), followed by the activation of various calcium-dependent proteins, including calmodulin (CaM), protein kinase C (PKC) and Akt/PKB (Right panel). Figure is adopted from [73].
4.2. Wound healing
Although most attention is given to endothelial primary cilia and NO with respect to vascular homeostasis, the interplay extends into the surrounding vascular smooth muscle cell (VSMC) layer. Research also indicates that all three isoforms of NOS may be present in VSMC depending on blood vessel type [52]. Under normal conditions, VSMC cilia extend toward the extracellular matrix. Interestingly, endothelial injury, such as a scratch wound, caused VSMC primary cilia migration to the wound edge where about 88% of the cilia observed where positioned with their long axis pointing toward the wound [53]. VSMC cilia have been shown to express polycystins as well as α3- and β1- integrins. When the integrin function was blocked, only about 30% of the cilia were positioned at the wound edge. This suggests that VSMC primary cilia may be involved in wound healing mediated by integrins. To further support primary cilia role in wound healing, studies using deciliated VSMC reported slower wound healing when subjected to scratch-wounding than ciliated control cells [53]. As the cilia in the wounded area are directly exposed to fluid shear stress from the blood flow, this might lead to the activation of the mechanosensory ciliary polycystin complex and an increase in intracellular calcium. This latter in turn can initiate vasoconstriction in VSMC, thus isolating the wounded area in order for the platelets to initiate clot formation. Once calcium reaches a certain level, calcium/calmodulin complex forms leading to the activation of both eNOS and nNOS, initiating vasodilation and promoting the next phase of wound healing. In another study, platelet derived growth factor receptor alpha (PDGFRα), a tyrosine kinase with a significant role in proliferation, was found to localize to fibroblast primary cilia when growth is arrested. Ligand activation of PDGFRα is known to activate the AKT and MEK/ERK proliferative pathways, alltogether suggesting primary cilia may play a role in wound healing and tissue homeostasis [54]. As the tissue begins to repair, the clot must be dissolved in order to maintain blood flow, generally considered clot retraction and platelet inhibition. NO, along with being a potent vasodilator is also known to inhibit platelet aggregation, secretion, adhesion, and fibrinogen binding through activation of guanylyl cyclase and cGMP and the inhibition of thromboxane A2. This mechanism reduces platelet aggregation and platelet “stickiness” enabling the clot to dissolve and the wound healing to complete [55–57]. Taken all the above together, an interplay between primary cilia function and NO could be implicated in wound healing and repair.
4.3. Dopamine signaling
Hypertension in PKD patients in late stages of the disease is exacerbated by an increased kidney volume but hypertension can be observed in children and early stages of the disease long before renal function deteriorates. There is evidence to suggest and increase in sympathetic activation that occurs in these patients independent of kidney function. Dopamine, an endogenous neuronal hormone with action in the sympathetic nervous system, is known to be involved in the regulation of blood pressure. Abnormalities in dopamine signaling can contribute to hypertensive states in humans. Dopamine 1-like receptors, D1 and D5, have both been found to localize to primary cilia [18, 58–60]. Currently, there are no pharmacological agents available that selectively target D1 or D5 but studies using agents selective for dopamine 1-like receptor subtypes have shown vasodilatory effects in peripheral arteries [61]. D5 is suggested to play both a chemosensory and mechanosensory role in primary cilia. Challenging endothelial ciliary knockout cells, pkd1−/− (no PC-1) and cilialess Tg737orpk/orpk with dopamine under static conditions resulted in a significantly less calcium influx than wildtype endothelial cells. This was attributed to smaller cilia in the knockout lines housing less D5 receptors in the cilia compared to the wildtype. Surprisingly, under flow conditions with dopamine, the mechanosensory function of the cilia knockout lines were restored compared to non-treated control knockouts. As calcium influxes in these cell lines are often associated with activation of eNOS, the results may imply a potential restoration of the lost vasodilatory responses caused by a failed ciliary induction of NO biosynthesis [58]. In addition, some evidence suggests that dopamine receptor 2 (D2) may also localize or be transported to the primary cilia [62]. Cerebral vasospasms were reversed when treated with dopamine; however, when haloperidol, a D2 selective antagonist, was administered, the vasorelaxation response was prevented. Additionally, it was reported that there was a significant increase in eNOS and iNOS expression after administration of dopamine which was also blocked by haloperidol [63]. It is possible that D2 is transported to the primary cilia under certain conditions to help mediate the activity of NOS within cells. Further support for the role of ciliary dopamine receptors in mediating NO can be found in clinical trials involving Autosomal Dominant Polycystic Kidney Disease (ADPKD) patients. ADPKD patients suffer from severe extrarenal manifestations mainly affecting the cardiovascular system such as hypertension. This could be due in part to the inability of primary endothelial cilia to properly respond to alterations in blood pressure and fail to initiate NO biosynthesis. In this study, flow mediated dilation of normotensive ADPKD patients was compared to that of healthy adults. The results indicated that ADPKD patients had markedly less dilation during sustained flow increases and a total loss of NO release compared to controls. When patients were given a brachial infusion of 0.25 to 0.5 ug/kg/min of dopamine, the results showed an upward trend in flow mediated dilation in ADPKD patients and reported a statistically significant increase in dilatory response at the highest dose [64]. Thus, dopamine receptors may facilitate, to some extent, a connection between primary cilia, NO, and blood pressure regulation in ADPKD.
4.4. Cell proliferation
Not only do primary cilia provide a sensory hub, they also play a regulatory role in cell proliferation. The ciliary structure extends from the basal body which is comprised of the joined mother and daughter centrioles [65, 66]. Cilia are observed in G0 and G1 stages in the cell cycle and are reabsorbed prior to mitotic entry. In cancerous tissues, cilia are not present on most proliferative cells suggesting that although cilia may not be directly involved in cell division, they do play a role in the entry and exit of mitosis [67–70]. Interestingly, NO has been proposed as a modulator of cellular proliferation as well and may have a complementary role alongside primary cilia. In most cases, NO has been shown to arrest the cell cycle preventing the transition from G1 to S phase. This inhibition of cell proliferation occurs in a dose dependent mechanism, the adequate concentrations of NO are caused by an increase in available L-arginine which is mediated by several cytokines. Interestingly, PC-1 has been shown to mediate the JAK/STAT pathway in several ways. Full length PC-1 is able to activate STAT3; when the cytosolic tail of PC-1 is cleaved upon cessation of luminal flow, it can coactivate STAT-1, −3, and −6 as well as JAK2. The PC-1 tail sensitizes the cells to cytokines and growth factor signaling exaggerating the cellular response and potentially leading to an increase in L-arginine thus arresting cell division [71, 72].
5. Conclusion
Both primary cilia and nitric oxide, independently, are essential for normal tissue functions. However, their functional roles and cellular pathways often parallel or complement one another. Although this review only touched on a small portion of possible connections, there is scattered literary evidence to suggest a linkage between the two in many more organ systems and cellular signaling pathways. Unfortunately, any research into the direct linkage between primary cilia and nitric oxide is far and few between. hence the aim of this review was to present a gap in our knowledge and initiate a discussion leading to a closer examination of the topics presented. Elucidating the connection between cilia and nitric oxide signaling would provide new insight into the cellular mechanisms that govern our bodies and thus the potential to better understand disease pathologies and provide new targets for therapies.
Acknowledgements
The authors apologize to those whose work is not cited in this paper. H. Saternos’ work partially fulfills the requirements for a Ph.D. degree in Pharmacology and Experimental Therapeutics. Work from our laboratory has been supported by grants from AHA (16SDG31330001) and NIH/NHLBI (1R15HL140523–01) to W.A.A. We are thankful to the University of Toledo research and sponsored programs as part of this work is also funded by The University of Toledo’s intramural startup fund for W.A.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veland IR, et al. , Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol, 2009. 111(3): p. p39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falk N, et al. , Specialized Cilia in Mammalian Sensory Systems. Cells, 2015. 4(3): p. 500–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters AM and Beales PL, Ciliopathies: an expanding disease spectrum. Pediatr Nephrol, 2011. 26(7): p. 1039–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AbouAlaiwi WA, et al. , Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res, 2009. 104(7): p. 860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauli SM, et al. , Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation, 2008. 117(9): p. 1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surya M Nauli HSH, AbouAlaiwi Wissam A., Lao Shao T., and Nauli Andromeda M., Primary Cilia are Mechanosensory Organelles in Vestibular Tissues in Mechanosensitivity and Mechanotransduction, Kamkin IKA, Editor. 2011, Springer Science + Business Media. [Google Scholar]
- 7.Buerk DG, Barbee KA, and Jaron D, Nitric oxide signaling in the microcirculation. Crit Rev Biomed Eng, 2011. 39(5): p. 397–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley SA and Steinert JR, Nitric Oxide-Mediated Posttranslational Modifications: Impacts at the Synapse. Oxid Med Cell Longev, 2016. 2016: p. 5681036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syed MA, et al. , Role of Nitric Oxide Isoforms in Vascular and Alveolar Development and Lung Injury in Vascular Endothelial Growth Factor Overexpressing Neonatal Mice Lungs. PLoS One, 2016. 11(1): p. e0147588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Vanhoutte PM, and Leung SW, Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci, 2015. 129(2): p. 83–94. [DOI] [PubMed] [Google Scholar]
- 11.Luiking YC, Engelen MP, and Deutz NE, Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care, 2010. 13(1): p. 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Majeed S, Moloney BC, and Nauli SM, Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci, 2012. 69(1): p. 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deane JA, et al. , Visualizing renal primary cilia. Nephrology (Carlton), 2013. 18(3): p. 161–8. [DOI] [PubMed] [Google Scholar]
- 14.Yuan X, Serra RA, and Yang S, Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann N Y Acad Sci, 2015. 1335: p. 78–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guemez-Gamboa A, Coufal NG, and Gleeson JG, Primary cilia in the developing and mature brain. Neuron, 2014. 82(3): p. 511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satir P, CILIA: before and after. Cilia, 2017. 6: p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechtreck KF, IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem Sci, 2015. 40(12): p. 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaf A and Von Zastrow M, Dopamine receptors reveal an essential role of IFT-B, KIF17, and Rab23 in delivering specific receptors to primary cilia. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhyay S, et al. , Trafficking to the primary cilium membrane. Mol Biol Cell, 2017. 28(2): p. 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malicki J and Avidor-Reiss T, From the cytoplasm into the cilium: bon voyage. Organogenesis, 2014. 10(1): p. 138–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng L, et al. , Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci, 2006. 119(Pt 7): p. 1383–95. [DOI] [PubMed] [Google Scholar]
- 22.Szymanska K and Johnson CA, The transition zone: an essential functional compartment of cilia. Cilia, 2012. 1(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, et al. , Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun, 2014. 5: p. 5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Q and Nelson WJ, Ciliary diffusion barrier: the gatekeeper for the primary cilium compartment. Cytoskeleton (Hoboken), 2011. 68(6): p. 313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klink BU, et al. , A recombinant BBSome core complex and how it interacts with ciliary cargo. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, et al. , BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci, 2013. 126(Pt 11): p. 2372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen VL, et al. , Whole-Organism Developmental Expression Profiling Identifies RAB-28 as a Novel Ciliary GTPase Associated with the BBSome and Intraflagellar Transport. PLoS Genet, 2016. 12(12): p. e1006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Pan J, and Snell WJ, Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell, 2006. 125(3): p. 549–62. [DOI] [PubMed] [Google Scholar]
- 29.Nachury MV, How do cilia organize signalling cascades? Philos Trans R Soc Lond B Biol Sci, 2014. 369(1650). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huangfu D, et al. , Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature, 2003. 426(6962): p. 83–7. [DOI] [PubMed] [Google Scholar]
- 31.Liem KF Jr., et al. , The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol, 2012. 197(6): p. 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang BQ, et al. , Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol, 2006. 291(3): p. G500–9. [DOI] [PubMed] [Google Scholar]
- 33.Engel BD, et al. , The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. J Cell Biol, 2012. 199(1): p. 151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, et al. , The role of ciliary trafficking in Hedgehog receptor signaling. Sci Signal, 2015. 8(379): p. ra55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorn KV, Hughes CE, and Rohatgi R, A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell, 2012. 23(4): p. 823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caparros-Martin JA, et al. , The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum Mol Genet, 2013. 22(1): p. 124–39. [DOI] [PubMed] [Google Scholar]
- 37.Pusapati GV, et al. , EFCAB7 and IQCE regulate hedgehog signaling by tethering the EVC-EVC2 complex to the base of primary cilia. Dev Cell, 2014. 28(5): p. 483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trouillon R, Biological applications of the electrochemical sensing of nitric oxide: fundamentals and recent developments. Biol Chem, 2013. 394(1): p. 17–33. [DOI] [PubMed] [Google Scholar]
- 39.Wu G and Morris SM Jr., Arginine metabolism: nitric oxide and beyond. Biochem J, 1998. 336 (Pt 1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forstermann U and Sessa WC, Nitric oxide synthases: regulation and function. Eur Heart J, 2012. 33(7): p. 829–37, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boo YC and Jo H, Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol, 2003. 285(3): p. C499–508. [DOI] [PubMed] [Google Scholar]
- 42.Su S, et al. , Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods, 2013. 10(11): p. 1105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauli SM, Pala R, and Kleene SJ, Calcium channels in primary cilia. Curr Opin Nephrol Hypertens, 2016. 25(5): p. 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delling M, et al. , Primary cilia are not calcium-responsive mechanosensors. Nature, 2016. 531(7596): p. 656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leonard PM, et al. , Crystal structure of the Lrp-like transcriptional regulator from the archaeon Pyrococcus furiosus. EMBO J, 2001. 20(5): p. 990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stahmann N, et al. , Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem, 2010. 285(14): p. 10638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siragusa M, et al. , Stromal cell-derived factor 2 is critical for Hsp90-dependent eNOS activation. Sci Signal, 2015. 8(390): p. ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, et al. , Differential effects of heat shock protein 90 and serine 1179 phosphorylation on endothelial nitric oxide synthase activity and on its cofactors. PLoS One, 2017. 12(6): p. e0179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen K, Pittman RN, and Popel AS, Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal, 2008. 10(7): p. 1185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi S and Mendelsohn ME, Calmodulin-dependent and -independent activation of endothelial nitric-oxide synthase by heat shock protein 90. J Biol Chem, 2003. 278(11): p. 9339–44. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi S and Mendelsohn ME, Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem, 2003. 278(33): p. 30821–7. [DOI] [PubMed] [Google Scholar]
- 52.Buchwalow IB, et al. , Vascular smooth muscle and nitric oxide synthase. FASEB J, 2002. 16(6): p. 500–8. [DOI] [PubMed] [Google Scholar]
- 53.Lu CJ, et al. , Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res, 2008. 31(3): p. 171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider L, et al. , PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol, 2005. 15(20): p. 1861–6. [DOI] [PubMed] [Google Scholar]
- 55.Wang GR, et al. , Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc Natl Acad Sci U S A, 1998. 95(9): p. 4888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du X, A new mechanism for nitric oxide–and cGMP-mediated platelet inhibition. Blood, 2007. 109(2): p. 392–393. [Google Scholar]
- 57.Riddell DR and Owen JS, Nitric oxide and platelet aggregation. Vitam Horm, 1999. 57: p. 25–48. [DOI] [PubMed] [Google Scholar]
- 58.Abdul-Majeed S and Nauli SM, Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension, 2011. 58(2): p. 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Upadhyay VS, et al. , Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front Physiol, 2014. 5: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marley A and von Zastrow M, DISC1 regulates primary cilia that display specific dopamine receptors. PLoS One, 2010. 5(5): p. e10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asghar M, et al. , Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep, 2011. 13(4): p. 294–302. [DOI] [PubMed] [Google Scholar]
- 62.Omori Y, et al. , Identification of G Protein-Coupled Receptors (GPCRs) in Primary Cilia and Their Possible Involvement in Body Weight Control. PLoS One, 2015. 10(6): p. e0128422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pyne-Geithman GJ, et al. , Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro. Neurocrit Care, 2009. 10(2): p. 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorthioir A, et al. , Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int, 2015. 87(2): p. 465–72. [DOI] [PubMed] [Google Scholar]
- 65.Lattao R, Kovacs L, and Glover DM, The Centrioles, Centrosomes, Basal Bodies, and Cilia of Drosophila melanogaster. Genetics, 2017. 206(1): p. 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearson CG, Choosing sides--asymmetric centriole and basal body assembly. J Cell Sci, 2014. 127(Pt 13): p. 2803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goto H, Inoko A, and Inagaki M, Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol Life Sci, 2013. 70(20): p. 3893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ke YN and Yang WX, Primary cilium: an elaborate structure that blocks cell division? Gene, 2014. 547(2): p. 175–85. [DOI] [PubMed] [Google Scholar]
- 69.Plotnikova OV, Golemis EA, and Pugacheva EN, Cell cycle-dependent ciliogenesis and cancer. Cancer Res, 2008. 68(7): p. 2058–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao M and Zhong Q, Cilia in autophagy and cancer. Cilia, 2015. 5: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Talbot JJ, et al. , Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A, 2011. 108(19): p. 7985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weimbs T, Olsan EE, and Talbot JJ, Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAKSTAT, 2013. 2(2): p. e23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saternos HC and AbouAlaiwi WA, Implications of Dysfunction of Mechanosensory Cilia in Polycystic Kidney Disease, in Polycystic Kidney Disease, Li X, Editor. 2015: Brisbane (AU). [PubMed] [Google Scholar]