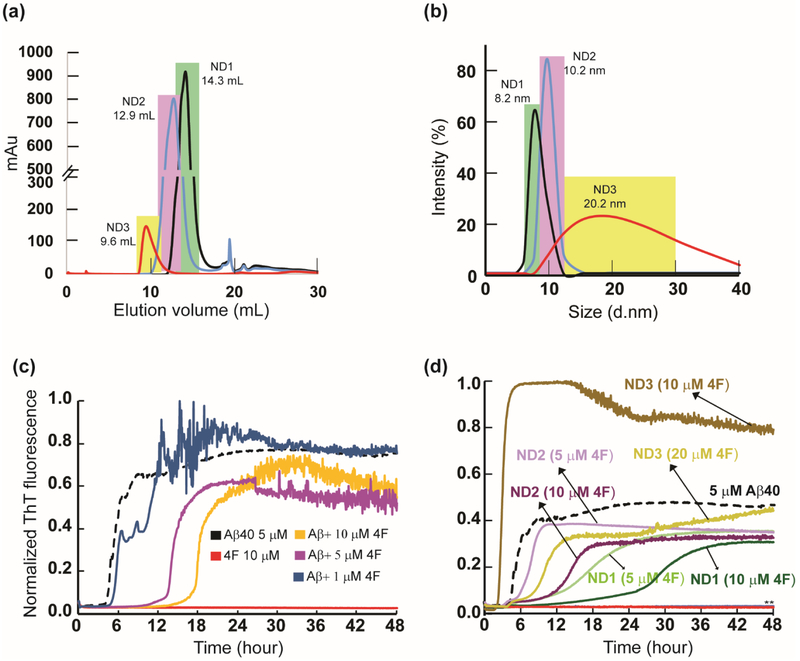
Fig. 1.
4F peptide (lipid-free or nanodisc-bound) delay amyloid aggregation of Aβ40. (a) Size-exclusion chromatography showing variable size 4F-encased DMPC nanodiscs at peptide:lipid (w/w) ratio of 1:1 (ND1), 1:2 (ND2) and 1:4 (ND3). (b) Size distribution profile of nanodiscs as a function of their hydrodynamic diameter measured using DLS. The highlighted colors in DLS indicate their corresponding SEC fractions. ThT fluorescence monitor the effect of 4F (lipid-free) peptide in solution (c) and 4F-encased lipid-nanodiscs (d) on Aβ40 aggregation (at 5 μM) kinetics at 37 °C in 10 mM sodium phosphate buffer (pH 7.4). The ThT fluorescence of Aβ40 aggregation in the absence of 4F is shown in black and the retardation of aggregation with an increasing concentration of 4F peptide is indicated with different colors. The double asterisks (**) marked in Fig. 1d indicate ND1 and ND2 (containing 20 μM 4F) abolish Aβ40 aggregation. None of the nanodiscs (ND1-3) bind to ThT (see Figure S1a). It should be noted that a change in the 4F-peptide concentration also indicates a corresponding change in the lipid concentration, and therefore an increase in the 4F-peptide concentration indicates an increase in the nanodiscs concentration in the sample.