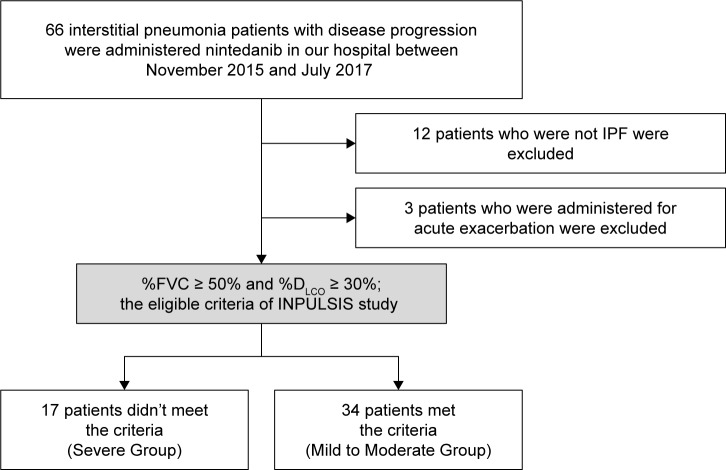
Figure 1.
Study flow chart.
Notes: Between November 2015 and July 2017, 66 patients received nintedanib at Chiba University Hospital. Of these 66 patients, 11 who did not have idiopathic pulmonary fibrosis (IPF) and three who received nintedanib for acute exacerbation were excluded. Patients who met one of the eligible criteria of the INPULSIS trial (% forced vital capacity [FVC] ≥ 50%; % diffusion capacity of the lung for carbon monoxide [DLCO] ≥ 30%) were classified as Mild to Moderate Group, and those who did not meet the criteria were classified as Severe Group.