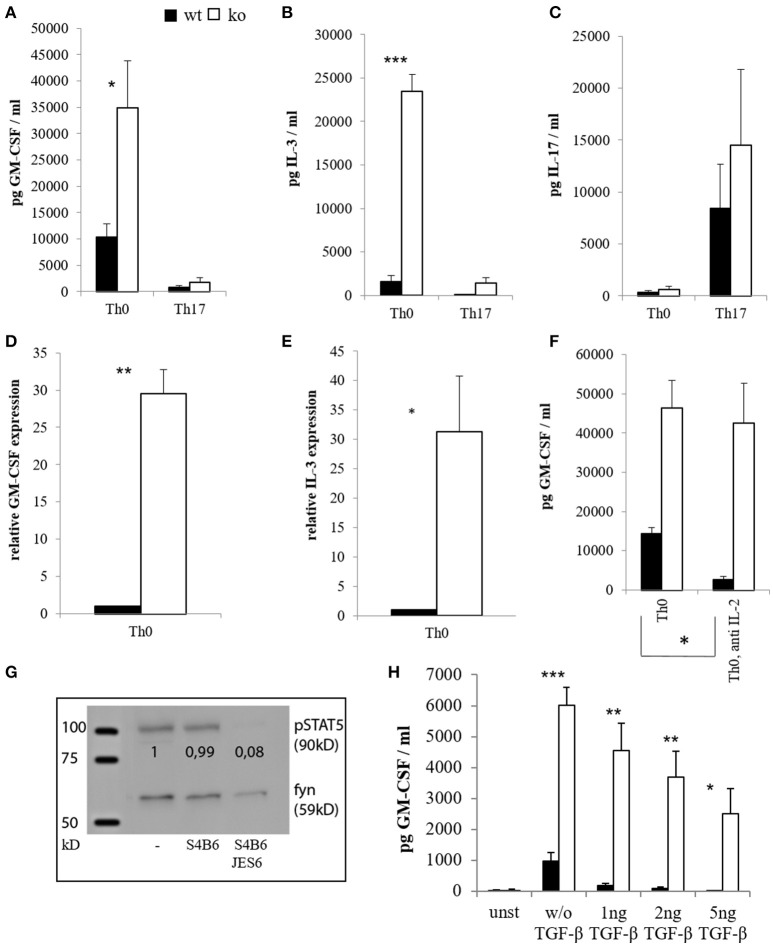
Figure 1.
Cytokine expression of CD4+ cells in vitro. CD4+ CD62L+ cells of wt and Cblb−/− mice were kept in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 10% FCS, 2 mM l-glutamine and penicillin-streptomycin [50 U/ml] and stimulated with 1 μg/ml anti-CD28 and 3 μg/ml platebound anti-CD3, with or without Th17 differentiation conditions (5 ng/ml TGF-β, 20 ng/ml IL-6, 10 ng/ml IL-23, 2 μg/ml antiIFN-γ, 2 μg/ml antiIL-4). GM-CSF [(A), n = 4–8; 3–6 independent experiments] and IL-3 levels [(B), n = 4–8; 3–6 independent experiments] were measured on day 3 in the cell culture supernatants. To validate Th17 differentiation, IL-17 was measured as well [(C), n = 4; 4 independent experiments]. RNA was extracted on day 2, and qRT-PCR for GM-CSF [(D), n = 6; 5 independent experiments] and IL-3 [(E), n = 6; 5 independent experiments] was performed. In some experiments, IL-2 blocking antibodies JES6 (30 μg/ml) and S4B6 (40 μg/ml) were added in combination (= anti IL-2), and GM-CSF levels were measured in the supernatants on day 3 [(F), n = 4; 3 independent experiments]. To validate the antibody function, cells were lysed, submitted to western blot and pSTAT5 was detected [(G), 1 out of 2 experiments]. The loading control was considered in the quantification. (H) (n = 4; 2 independent experiments) shows GM-CSF amounts in supernatants on day 3 of unstimulated (= unst) or stimulated CD4+ cells treated with different amounts of TGF-β.