Abstract
Background
Resistant starches type 4 (RS4) are chemically modified starches that are resistant to digestion by human enzymes.
Objective
We aimed to test our hypothesis that replacement of standard starch with RS4 in a baked breakfast bar would decrease postprandial glycemic and insulinemic responses in healthy adults.
Methods
In this double-blind, randomized crossover study, 21 healthy adults [10 men; 20–45 y old; BMI (kg/m2): 19.3–27.0] consumed a baked breakfast bar containing tapioca-based RS4 (Actistar 75330; Cargill, Inc.) or a macronutrient-matched control bar, delivering 32 g and 4 g of dietary fiber, respectively. Primary outcome was the incremental area under the curve (iAUC0–120 min) for postprandial capillary glucose. Other outcomes included postprandial serum insulin iAUC0–120 min, glucose and insulin maximum concentration (Cmax), and time to Cmax (Tmax).
Results
Median glucose iAUC0–120 min was 22% lower (P < 0.05) and median insulin iAUC0–120 min was 37% lower (P < 0.05) after consumption of the RS4 food compared with the control food. Glucose and insulin Cmax and Tmax were not significantly different (P > 0.05) between foods.
Conclusion
The results suggest that replacement of standard starch with tapioca-based RS4 is a practical approach for reducing available carbohydrate in products and achieving postprandial blood glucose management. This trial was registered at clinicaltrials.gov as NCT03239288.
Keywords: fiber, clinical trial, human, tapioca, Actistar, resistant starch
Introduction
Resistant starches (RSs) are indigestible by mammalian enzymes (1) and can be classified into 5 subtypes: RS1, starch granules embedded in indigestible plant material such as whole grains; RS2, native granular starch such as raw potato or high-amylose maize or wheat; RS3, crystallized starch made by alternative cooking and cooling; RS4, chemically modified starch typically through esterification, cross-linking, or transglycosylation; and RS5, fatty acid and amylose interactions such as palmitic acid-amylose complex (2). RS is an important functional ingredient for the food industry owing to its desirable physicochemical properties (1) such as swelling, viscosity increase, gel formation, and water-binding capacity, providing many advantages over traditional fibers, and thus may be utilized in many products (3). In addition, a number of beneficial physiologic effects have been associated with RS including regulation of blood cholesterol levels, attenuation of fasting and postprandial glucose, improved laxation/bowel function, and reduced energy intake (4).
The majority of published nutrition and health research on RS has focused on the RS2 and RS3 components. For example, the recent preliminary draft review conducted by the FDA on beneficial physiologic effects of putative fibers only identified 5 studies with RS4; of these only 2 were determined initially to meet the criteria for consideration as acceptable evidence, and the final determination on the supporting science has not been concluded by the FDA as of April 2018. Of these 2 studies, only 1 examined the effect of RS4 (wheat-based) on postprandial glucose (5). Although all RS components are resistant to enzymatic degradation, their effect on blood glucose is dependent on type (6) and possibly the type of chemical modification (7), as in the case for RS4. Thus, clinical trials on specific RS preparations are necessary to confirm the beneficial physiologic effects.
Actistar 75330 is a phosphorylated resistant food starch derived from tapioca that is commercially available from Cargill, Inc. In addition to the study on wheat-based RS4 (5) referenced by the FDA in their preliminary review (4), there are several others that investigated the effects of phosphorylated RS4 derived from different plant sources on postprandial glucose and insulin responses. Of these, only 1 study investigated the effect of phosphorylated RS4 derived from tapioca (8). In this study, postprandial glucose was lower after consumption of highly cross-linked starch phosphate tapioca RS4 dissolved in water compared with solutions containing unmodified tapioca starch, and microcrystalline cellulose, matched for total carbohydrates (30 g) (8).
Owing to its versatility, RS4 is typically incorporated alongside other ingredients into a final food product, making it important to investigate the effect of a solid food product containing tapioca RS4. In addition, the specific effect of tapioca-based RS4 on postprandial insulin responses has, to our knowledge, never been investigated. Thus, the objective of the present study is to assess the acute postprandial glucose and insulin responses to a baked breakfast bar containing RS4 in the form of distarch phosphate from tapioca (Actistar 75330) in healthy adults.
Methods
Study design
The study (NCT03239288) was conducted at Biofortis, Addison, IL, between June 2017 and August 2017. The clinical study protocol and relevant documents were approved by an institutional review board (IntegReview, Austin, TX) before initiation of the trial, and subjects provided written informed consent and authorization for disclosure of protected health information before enrolling in the study. All subjects received monetary compensation for their participation. This study was conducted consistent with appropriate Good Clinical Practice Guidance, the Declaration of Helsinki (2006), and the United States 21 Code of Federal Regulations. Clinical on-site risk-based subject monitoring was performed on critical processes and data including subject eligibility, informed consent administration, study product dispensation, safety assessments, and study endpoints. In addition, best clinical data management practices for data quality were conducted, including 100% validation of the primary study endpoint.
The study was a randomized, controlled, double-blind, crossover study, with 2 postprandial visits and a 4- to 7-d washout between the visits. Subjects were instructed to refrain from vigorous activity, consume a diet with a minimum of 150 g carbohydrate, and maintain their habitual fluid consumption for the 24 h before each of the test visits. Subjects arrived for each visit in a fasted (12–14 h) state, at which time the single-meal challenge test was conducted. The challenge test interventions were a baked breakfast bar containing 27 g of the tapioca-based RS4 (Actistar 75330 from Cargill, Inc.) or a control bar that was matched for total fat, protein, and total carbohydrate content. The breakfast bars were consumed with 8 oz (237 mL) of water. During the remainder of the 2-h challenge test, subjects were allowed ≤16 oz (474 mL) of water but no other food or beverage.
A statistician generated a block (blocking size of 4) randomization list for a 2-treatment, 2-period, 2-sequence, crossover design with the use of the SAS PROC PLAN with a 1:1 allocation ratio. Numbered, opaque, and sealed envelopes concealing the allocation sequence were opened sequentially by an independent staff member only after a subject was confirmed eligible for the study, and the randomization number/test sequence was recorded with the subject's source documentation. The study subjects, investigators, and outcome assessor were blinded to the intervention.
Study products
The RS4 and control products were baked breakfast bars matched for fat, protein, and total carbohydrate (Table 1). The RS4 bar contained Actistar 75330, a phosphorylated resistant food starch derived from tapioca (Cargill, Inc.) that provides 88.6% (wt:wt) dietary fiber as analyzed by the AOAC 991.43 method. In place of the RS4, the control bar contained standard corn starch (0% dietary fiber per the AOAC 991.43 method). Briefly, RS4 or corn starch and ingredients common to both bars (water, all-purpose flour, granulated sugar, palm shortening, honey, vital wheat gluten, soy lecithin, cinnamon, salt, baking soda, and natural flavorings) were mixed with the use of a Hobart N50 Commercial Mixer (Troy, OH). The dough was held at room temperature for 10 min. The dough was sheeted on a Rondo Sheeter Model SS067 (Moonachie, NJ) to a half-inch (12.7 mm) height and cut into 2-in (50.8 mm) squares. The squares were baked for 15 min at 400°F (204°C). After baking, the RS4 bars had a lighter brown color than the control bars, but both exhibited a firm outer crust and a soft interior (Supplemental Figure 1). During product formulation, the RS4 bars were observed to have a drier and more crumbly texture than the control bars. The squares were allowed to cool, then packaged and frozen until use. The RS4 breakfast bar contained 32 g fiber and the control breakfast bar, which included corn starch, contained 4 g fiber (verified in final products via the AOAC 991.43 method for dietary fiber).
TABLE 1.
Nutrient composition of breakfast bars1
| Per serving | Control | RS4 |
|---|---|---|
| Energy, kcal | 310 | 202 |
| Total fat, g | 10 | 10 |
| Saturated fat, g | 5 | 5 |
| trans fat, g | 0 | 0 |
| Protein, g | 4 | 4 |
| Total carbohydrate, g | 55 | 56 |
| Dietary fiber, g | 4 | 32 |
Fat content was analyzed with the use of GC (AOAC 996.06), protein content was analyzed by the Dumas method (AOAC 992.15; AACC 46-30), and dietary fiber was analyzed by the AOAC 991.43 method. Calories were determined through calculation. RS4, resistant starch type 4.
Subjects
Eligible subjects were healthy men and women (nonpregnant, nonlactating) 20–45 y of age, inclusive, with BMI (in kg/m2) ≥18.5 and ≤26.9, and fasting blood glucose ≤110 mg/dL. Individuals who were from the surrounding area and met initial age and BMI requirements were contacted for their interest in participating in this study. Interested individuals were invited to a screening visit for study staff to determine their eligibility. Medical history and a standard chemistry and hematology profile were obtained at screening to rule out the presence of conditions that might affect the outcome of the study, including cardiovascular disease, diabetes mellitus, and uncontrolled hypertension. Individuals who reported taking medications that might alter carbohydrate metabolism, or weight-loss drugs or being on weight-loss programs within 4 wk of their screening visits were not included. Subjects were instructed to maintain their physical activity patterns, body weight, and habitual diet (including use of vitamins, minerals, and other supplements) throughout the trial. Subjects were also instructed to replicate their diet during the 24 h before each postprandial visit.
Laboratory measurements
Capillary blood glucose and intravenous blood insulin were measured at t = −15, −5, 15, 30, 45, 60, 90, and 120 min, where t = 0 min was the start of the study product consumption. Capillary glucose concentration during screening and both postprandial visits was assessed with the use of the Accu-Check Performa (Roche, Indianapolis, IN) finger-stick glucometer according to manufacturer's instructions, and with test strips from the same lot. A flexible catheter was inserted in the antecubital vein of the left arm and blood samples were collected into serum separator tubes. After separation by centrifugation (10 min, 1600 × g, 12°C), serum was divided into aliquots and then stored immediately at −80°C before shipping to Quest Diagnostics Laboratory (Wooddale, IL) for insulin measurement with Access Ultrasensitive Insulin assay via Access Immunoassay Systems on the UniCel DxI 800 (Beckman Coulter).
Statistical analysis
All statistical analyses were conducted with the use of SAS for Windows (version 9.4; SAS Institute, Cary, NC). Sample size calculations were performed based on the composition of the study products, a published reference study (9), and a study conducted by Biofortis (unpublished). A sample of 19 subjects was expected to provide 80% power (α = 0.05, 2-tailed) to detect a 39% difference in glucose incremental AUC (iAUC) between study products. A sample of 22 subjects was randomly assigned to allow for attrition and noncompliance.
Primary analysis was completed for the intent-to-treat sample, which included all subjects who were randomly assigned into the study. In addition, analyses were conducted on subjects who completed both intervention periods and were compliant to the overall diet [per protocol (PP)]. All analyses of study samples were identified before locking the database. The statistician performing the analysis and scientific investigators remained blinded to the intervention sequence until after the completion of all statistical analyses. No qualitative differences were found between the intent-to-treat and PP populations; therefore, the results for the PP population are reported herein.
Unless otherwise stated, tests of significance were 2-sided and performed at a 5% level of significance. The primary outcome variable was the iAUC0–120 min for capillary glucose. Other outcome variables included iAUC0–120 min for insulin, maximum concentration (Cmax) for glucose and insulin, and time to Cmax (Tmax) for glucose and insulin. Outcome variables were assessed via repeated-measures ANCOVA through the SAS PROC MIXED procedure. Initial repeated-measures ANCOVA models contained terms for intervention, intervention sequence, and intervention period, in which intervention was specified as a repeated effect and subject was nested within sequence. Models were reduced via a backward selection method until only significant terms or intervention remained.
The assumption of normality of residuals was investigated for each response measurement with the use of the Shapiro-Wilk test. If the normality assumption was rejected at the 5% level of significance, then analysis based on rank-transformed data was performed. Finally, missing data were not imputed; thus, only observed data were analyzed. Data are presented as means ± SDs or medians (95% CIs).
Results
Subjects
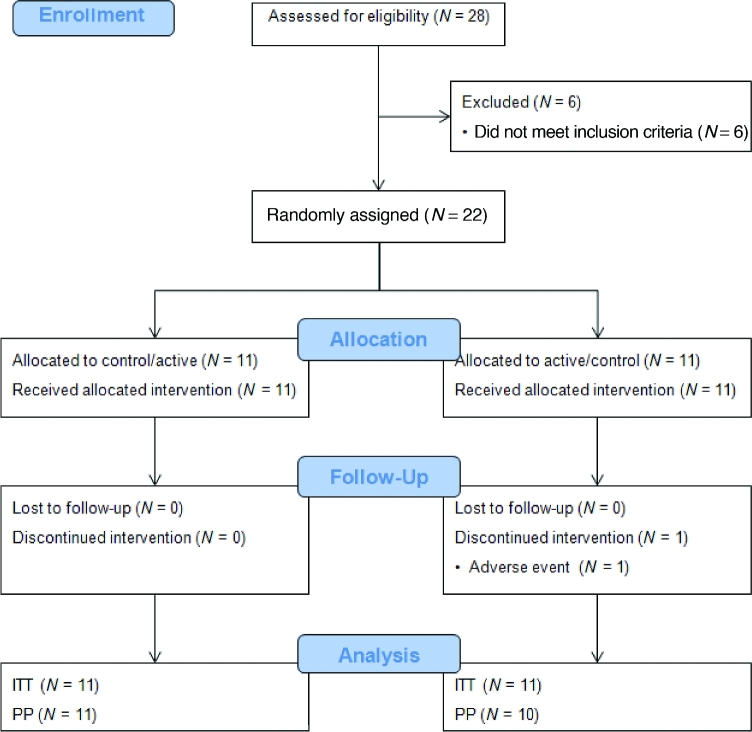
Of the 28 subjects screened, 22 met the inclusion criteria and were randomly assigned (Figure 1) to 1 of 2 sequences: control/RS4 (n = 11) or RS4/control (n = 11). One subject withdrew from the study after completing 1 intervention because of an adverse event (vasovagal) that was determined to not be related to the intervention by the clinical investigator, and was excluded from the PP sample. Overall, there were no adverse events determined to be related to the acute consumption of a study product, suggesting that the RS4 product was well-tolerated. Demographics and selected baseline characteristics of subjects are shown in Table 2. Subjects were between 20 and 45 y old with BMI within the healthy and overweight categories (19.3–27.0) and had blood pressure and fasting glucose levels that were within healthy ranges.
FIGURE 1.
CONSORT diagram indicating sample sizes at each stage during the study. CONSORT, Consolidated Standards of Reporting Trials; ITT, intent to treat; PP, per protocol.
TABLE 2.
Subject demographics and selected characteristics (n = 21)1
| Variable | |
|---|---|
| Female sex, n (%) | 11 (52.4) |
| Age, y | 34.14 ± 8.62 |
| Race, n (%) | |
| White | 15 (71.4) |
| Black or African American | 1 (4.8) |
| Asian or Pacific Islander | 5 (23.8) |
| Weight, kg | 67.97 ± 10.68 |
| BMI, kg/m2 | 23.50 ± 2.32 |
| Systolic blood pressure, mm Hg | 109.90 ± 11.05 |
| Diastolic blood pressure, mm Hg | 66.57 ± 9.37 |
| Fasting glucose, mg/dL | 94.00 ± 10.19 |
Values are means ± SDs for quantitative data and n (%) for categoric data, and included subjects in the per protocol sample.
Postprandial glucose and insulin responses
Median glucose iAUC0–120 min was 22% lower (P = 0.01) after the consumption of the RS4 breakfast bar compared with the control (Table 3). Similarly, median insulin iAUC0–120 min was significantly lower (37% reduction, P = 0.03) after the RS4 breakfast bar compared with the control. These decreases in iAUC0–120 min for glucose and insulin were not accompanied by significant changes in Cmax and Tmax. Postprandial capillary glucose and serum insulin concentrations over the 2-h postprandial period are shown in Figure 2A, B.
TABLE 3.
Postprandial glucose and insulin responses1
| Control | RS4 | P 2 | |
|---|---|---|---|
| Glucose iAUC0–120 min, mg/dL × min | 1651.08 (1488.77, 2519.50) | 1281.31 (470.13, 1921.80) | 0.0135 |
| Glucose Cmax, mg/dL | 127.00 (116.00, 137.00) | 121.00 (115.00, 133.00) | 0.1653 |
| Glucose Tmax, min | 41.00 (40.00, 49.00) | 40.00 (28.00, 42.00) | 0.0920 |
| Insulin iAUC0–120 min, µIU/mL × min | 2544.25 (2026.89, 3172.10) | 1610.57 (907.22, 2852.45) | 0.0299 |
| Insulin Cmax, µIU/mL | 51.70 (40.60, 61.30) | 40.20 (34.80, 60.00) | 0.1516 |
| Insulin Tmax, min | 30.00 (26.00, 42.00) | 30.00 (26.00, 43.00) | 0.9797 |
Values are medians (95% CIs). Results shown here are from the per protocol population (n = 20). Cmax, maximum concentration; iAUC, incremental AUC; RS4, resistant starch type 4; Tmax, time to maximum concentration.
Between-group differences were assessed via repeated-measures ANCOVA whereby initial models contained terms for intervention, intervention sequence, and intervention period. Models were reduced via a backward selection method until only significant terms or intervention remained in the model. P values for glucose iAUC0–120 min, glucose Tmax, insulin iAUC0–120 min, insulin Cmax, and insulin Tmax were obtained from the final ANCOVA model which only contained intervention. P value for glucose Cmax was obtained from the final ANCOVA model, which contained test period and intervention.
FIGURE 2.
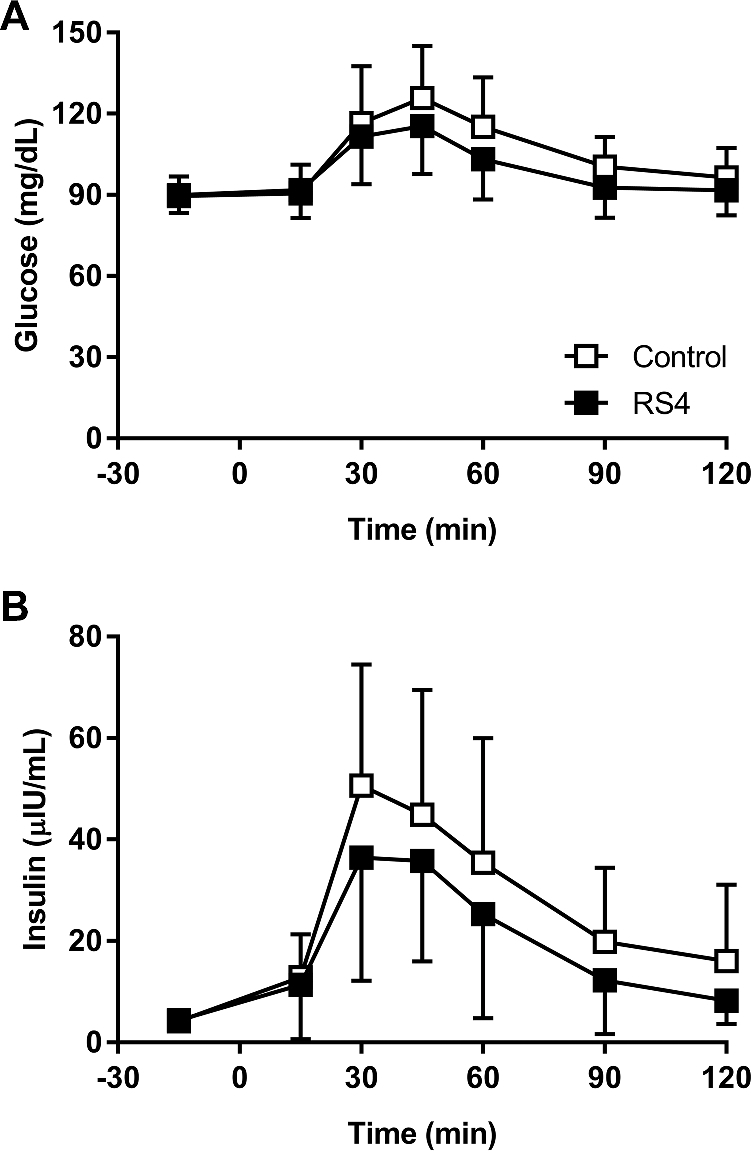
Capillary glucose (A) and serum insulin (B) concentrations in healthy adults (n = 21) who consumed a breakfast bar containing RS4 or a control bar that was matched for fat, protein, and total carbohydrate content, delivering 32 g and 4 g of dietary fiber, respectively. RS4, resistant starch type 4.
Discussion
The rate of digestion of foods containing a higher proportion of RS relative to available carbohydrates in the small intestine is much slower when compared with food containing only available carbohydrates (10), thus resulting in lower postprandial glucose concentrations (11). Consistent with this, we demonstrated a reduction in postprandial glucose response after replacement of 52.6% of carbohydrate from standard starch with carbohydrate from RS4. To the best of our knowledge, only 1 other study has to date examined the effect of replacing carbohydrate with RS4 derived from tapioca on postprandial glucose (8). In that study, consumption of a solution containing 30 g highly cross-linked starch phosphate derived from tapioca starch (providing 29.8 g fiber) by healthy men (n = 10) resulted in reductions in plasma glucose compared with a solution containing 30 g unmodified tapioca starch (<0.1 g fiber) (8). Our study complements this previous study by examining the effect of another tapioca-based RS4, but incorporated into a solid food matrix containing carbohydrates and other nutrients. In addition, we are the first, to our knowledge, to demonstrate that RS4 derived from tapioca not only lowers postprandial glucose, but also attenuates postprandial insulin when used to replace available carbohydrate in solid foods.
The glucose-lowering effect after substitution of standard starch with RS4 observed in our study is consistent with that reported by others with different types of RS4. Postprandial glucose was lower after consumption of a solution containing 38 g of distarch phosphate potato RS4 compared with a solution of 50 g dextrose, but not when 38 g of the distarch phosphate potato RS4 combined with 50 g dextrose was compared with 50 g dextrose alone (12). Consumption of cookies containing distarch phosphate potato RS4 (providing 24 g fiber) resulted in lower postprandial glucose and insulin responses compared with a low-fiber control (0.5 g fiber), matched for total carbohydrates (37 g) (9). Consumption of a wheat-based RS4 dissolved in water resulted in lower postprandial glucose compared with a dextrose solution, matched for total carbohydrates (30 g) (6). Finally, acid-hydrolyzed and heat-treated maize RS4 incorporated into baked muffins and scones were recently shown to elicit lower postprandial glucose and insulin responses than low-fiber controls with similar total carbohydrate contents (13, 14).
As demonstrated by the aforementioned studies, replacement of available carbohydrate by RS4 lowers postprandial glucose because the amount of carbohydrate contributing to blood glucose is now reduced in the food product. However, 1 study reported lower postprandial glucose and insulin responses after consumption of bars containing phosphated distarch phosphate RS4 derived from wheat (providing 20 g fiber) compared with a low-fiber bar (5 g fiber) matched for total available carbohydrates (51 g) (5). Studies on RS in animals show decreased gastrointestinal transit time of available carbohydrates provided with RS2 (15), which may allow portions of the available carbohydrate to escape digestion and absorption in the small intestine. This is a possible mechanism for the reduction of postprandial glycemia by RS4 that should be investigated in the future. In addition, chronic (≥3 wk) periods of RS4 consumption were shown to alter gut microbiota composition and induced SCFA production (16–18), which are suggested to regulate glucose metabolism (19). Further studies are needed to better define the mechanisms by which chronic and acute RS4 consumption regulate postprandial and chronic glucose when RS4 is added to foods, independent of changes in available carbohydrate content.
This study only investigated the effects of replacing digestible carbohydrates with RS4. Future research should examine if the reduction in postprandial glucose and insulin persists when test foods containing RS4 and control foods are matched for available carbohydrates. This study is also limited in that it only examined the effects in healthy adults, and the effects in individuals with compromised carbohydrate and/or glucose regulation may differ. Finally, the intervention visits were not scheduled around the menstrual cycle for female subjects, but evidence on the menstrual cycle affecting postprandial glucose is inconsistent (20). The menstrual cycle has not been considered in other similar studies (6, 9, 12), and data are more generalizable to the total population when timing of the menstrual cycle is not considered. Similarly, subjects with BMI ranging from normal to overweight were included in the study to make the results more generalizable.
In addition to improving postprandial blood glucose, wheat- and potato-based RS4 consumption has been shown to impart other physiologic effects, including improvements in blood lipids and body composition (21). These effects were observed beyond the first 2 h of acute RS4 consumption and after long-term intakes, and thus cannot be investigated in our study on tapioca-based RS4. In addition, the effect of acute and chronic intake of tapioca-based RS4 on glucose metabolism in individuals with impaired glucose metabolism, such as those with diabetes, is unknown and warrants investigation.
To the best of our knowledge, this randomized, crossover, controlled study is the first report of the effect of Actistar 75330 RS4, a phosphorylated resistant food starch derived from tapioca, incorporated into a solid, baked food product, on postprandial glycemic and insulinemic responses in healthy adults. The only other study on tapioca-based RS4 measured postprandial glucose, but not insulin, after consumption of the RS4 dissolved in water only. The results suggest that replacement of standard starch with RS4 derived from tapioca is a promising strategy for postprandial blood glucose management.
Supplementary Material
Acknowledgments
This study was supported by Cargill, Inc. and conducted by Biofortis, Inc. We thank Libertie Mantilla for statistical analysis and consultation. The authors’ responsibilities were as follows—VG-C and EM: designed the research; EM and DL: conducted the research; VG-C: provided the study product; and all authors: wrote the manuscript, had primary responsibility for final content, and read and approved the final manuscript.
Notes
Supported by Cargill, Inc. to Biofortis, Mérieux NutriSciences.
Author disclosures: VG-C is employed by Cargill, Inc.; EM and DL are employees of Biofortis, Mérieux NutriSciences.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used:
- Cmax
maximum concentration
- iAUC
incremental area under the curve
- PP
per protocol
- RS
resistant starch
- Tmax
time to maximum concentration
References
- 1. Raigond P, Ezekiel R, Raigond B. Resistant starch in food: a review. J Sci Food Agric 2015;95(10):1968–78. [DOI] [PubMed] [Google Scholar]
- 2. Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M, et al. Resistant starch: promise for improving human health. Adv Nutr 2013;4(6):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charalampopoulos D, Wang R, Pandiella SS, Webb C. Application of cereals and cereal components in functional foods: a review. Int J Food Microbiol 2002;79(1–2):131–41. [DOI] [PubMed] [Google Scholar]
- 4. Office of Nutrition and Food Labeling, Center for Food Safety and Applied Nutrition, Food and Drug Administration, US Department of Health and Human Services Science review of isolated and synthetic non-digestible carbohydrates. 2016. [Internet]. [cited 2018 Jan 30]. https://www.fda.gov/downloads/Food/LabelingNutrition/UCM529049.pdf. [Google Scholar]
- 5. Al-Tamimi EK, Seib PA, Snyder BS, Haub MD. Consumption of cross-linked resistant starch (RS4XL) on glucose and insulin responses in humans. J Nutr Metab 2010:651063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haub MD, Hubach KL, Al-Tamimi EK, Ornelas S, Seib PA. Different types of resistant starch elicit different glucose responses in humans. J Nutr Metab 2010:230501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nabeshima EH, Bustos FM, Hashimoto JM, El Dash AA. Improving functional properties of rice flours through phosphorylation. Int J Food Prop 2010;13(4):921–30. [Google Scholar]
- 8. Tachibe M, Ohga H, Nishibata T, Ebihara K. Digestibility, fermentability, and energy value of highly cross-linked phosphate tapioca starch in men. J Food Sci 2011;76(6):H152–5. [DOI] [PubMed] [Google Scholar]
- 9. Stewart ML, Zimmer JP. A high fiber cookie made with resistant starch type 4 reduces post-prandial glucose and insulin responses in healthy adults. Nutrients 2017;9(3):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA. Resistant starch as functional ingredient: a review. Food Res Int 2010;43(4):931–41. [Google Scholar]
- 11. Wong THT, Louie JCY. The relationship between resistant starch and glycemic control: a review on current evidence and possible mechanisms. Starch 2017;69(7–8):1600205. [Google Scholar]
- 12. Haub MD, Louk JA, Lopez TC. Novel resistant potato starches on glycemia and satiety in humans. J Nutr Metab 2012:478043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart ML, Wilcox ML, Bell M, Buggia MA, Maki KC. Type-4 resistant starch in substitution for available carbohydrate reduces postprandial glycemic response and hunger in acute, randomized, double-blind, controlled study. Nutrients 2018;10(2):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart ML, Zimmer JP. Post-prandial glucose and insulin response to high-fiber muffin top containing resistant starch type 4 in healthy adults: a double-blind, randomized, controlled trial. Nutrition 2018;53:59–63. [DOI] [PubMed] [Google Scholar]
- 15. Ferguson LR, Tasman-Jones C, Englyst H, Harris PJ. Comparative effects of three resistant starch preparations on transit time and short-chain fatty acid production in rats. Nutr Cancer 2000;36(2):230–7. [DOI] [PubMed] [Google Scholar]
- 16. Dahl WJ, Ford AL, Ukhanova M, Radford A, Christman MC, Waugh S, Mai V. Resistant potato starches (type 4 RS) exhibit varying effects on laxation with and without phylum level changes in microbiota: a randomised trial in young adults. J Funct Foods 2016;23:1–11. [Google Scholar]
- 17. Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 2010;5(11):e15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Upadhyaya B, McCormack L, Fardin-Kia AR, Juenemann R, Nichenametla S, Clapper J, Specker B, Dey M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci Rep 2016;6:28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013;54(9):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol Rev 2014;36:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nichenametla SN, Weidauer LA, Wey HE, Beare TM, Specker BL, Dey M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol Nutr Food Res 2014;58(6):1365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.