Abstract
Key points
Re‐sensitization of P2X4 receptors depends on a protonation/de‐protonation cycle
Protonation and de‐protonation of the receptors is achieved by internalization and recycling of P2X4 receptors via acidic compartments
Protonation and de‐protonation occurs at critical histidine residues within the extracellular loop of P2X4 receptors
Re‐sensitization is blocked in the presence of the receptor agonist ATP
Abstract
P2X4 receptors are members of the P2X receptor family of cation‐permeable, ligand‐gated ion channels that open in response to the binding of extracellular ATP. P2X4 receptors are implicated in a variety of biological processes, including cardiac function, cell death, pain sensation and immune responses. These physiological functions depend on receptor activation on the cell surface. Receptor activation is followed by receptor desensitization and deactivation upon removal of ATP. Subsequent re‐sensitization is required to return the receptor into its resting state. Desensitization and re‐sensitization are therefore crucial determinants of P2X receptor signal transduction and responsiveness to ATP. However, the molecular mechanisms controlling desensitization and re‐sensitization are not fully understood. In the present study, we provide evidence that internalization and recycling via acidic compartments is essential for P2X4 receptor re‐sensitization. Re‐sensitization depends on a protonation/de‐protonation cycle of critical histidine residues within the extracellular loop of P2X4 receptors that is mediated by receptor internalization and recycling. Interestingly, re‐sensitization under acidic conditions is completely revoked by receptor agonist ATP. Our data support the physiological importance of the unique subcellular distribution of P2X4 receptors that is predominantly found within acidic compartments. Based on these findings, we suggest that recycling of P2X4 receptors regulates the cellular responsiveness in the sustained presence of ATP.
Keywords: P2X receptor, purinergic receptor, membrane trafficking, receptor re-sensitization, ATP, Patch clamp
Key points
Re‐sensitization of P2X4 receptors depends on a protonation/de‐protonation cycle
Protonation and de‐protonation of the receptors is achieved by internalization and recycling of P2X4 receptors via acidic compartments
Protonation and de‐protonation occurs at critical histidine residues within the extracellular loop of P2X4 receptors
Re‐sensitization is blocked in the presence of the receptor agonist ATP
Introduction
P2X receptors are a family of cation‐permeable, ligand‐gated ion channels that open in response to the binding of extracellular ATP. Seven mammalian P2X receptor subtypes (P2X1 to P2X7) have been identified as giving rise to various functional P2X receptors formed as homo‐ or heterotrimers (North, 2002). P2X receptors are widely but specifically distributed among different tissues and mediate a large variety of responses, including fast synaptic transmission in the central nervous system, contraction of smooth muscle cells, platelet aggregation, macrophage activation, cell proliferation and cell death (Surprenant & North, 2009; Burnstock et al. 2010; Kaczmarek‐Hajek et al. 2012). Based on the widespread implication of P2X receptor signalling in organ function under healthy and diseased conditions, P2X receptors represent important drug targets (Khakh & North, 2006). Exhaustive efforts have been undertaken to understand the molecular, pharmacological and functional properties of these receptors (Kaczmarek‐Hajek et al. 2012).
The gating of P2X receptors usually consists of a rapid activation phase upon binding of ATP, a desensitization phase and a relatively rapid deactivation phase upon removal of ATP. The main difference between receptors is their sensitivity for agonists and their activation and desensitization rates (Coddou et al. 2011). The extent of desensitization is of great physiological relevance because it determines the time course of P2X receptor signal transduction and regulates the responsiveness in the sustained presence of ATP. Subsequent re‐sensitization is required to return the receptor into its resting state. Therefore, desensitization and re‐sensitization are crucial determinants of P2X receptor signal transduction and responsiveness to ATP. The molecular mechanisms controlling desensitization and re‐sensitization are not yet fully understood. Desensitization probably represents a ligand‐bound, closed state of the receptor and the re‐sensitization process probably requires agonist unbinding and a conformational change to return the receptor into its resting state (Kaczmarek‐Hajek et al. 2012). Also, the agonist used to activate the receptor, agonist unbinding kinetics and/or calcium might influence the kinetics of desensitization and re‐sensitization. However, none of the proposed mechanisms fully explains all of the observed effects on receptor re‐sensitization, suggesting that additional regulatory processes must be involved (Roberts et al. 2006).
More recently, it has become apparent that cellular trafficking also affects receptor signalling. Some P2X receptors are predominantly expressed on intracellular organelles. Therefore, regulation of surface expression and activation by extracellular ATP might be controlled by trafficking of the receptor to and from the plasma membrane (Robinson & Murrell‐Lagnado, 2013). The physiological role of these dynamics remains to be explored but, in addition to regulating receptor density at the cell surface, they may also accelerate recovery from desensitization (Xu et al. 2014). P2X1 receptors rapidly desensitize in the presence of agonist and inhibiting the internalization and recycling of receptors reduces the rate of recovery from desensitization. (Lalo et al. 2012; Robinson & Murrell‐Lagnado, 2013). Similarly, P2X4 receptors undergo rapid constitutive internalization from the plasma membrane following activation and inhibition of dynamin‐mediated endocytosis slows the re‐sensitization process of P2X4 receptors (Robinson & Murrell‐Lagnado, 2013). In addition, P2X4 receptors are predominantly localized on late endosomal, lysosomal and/or lysosome‐related organelles (Bobanovic et al. 2002; Miklavc et al. 2011a; Qureshi et al. 2007). The physiological role of this stable intracellular expression in acidic compartments has been a long‐standing puzzle because the acidic pH inside these organelles inhibits receptor activation (Stoop et al. 1997; Wildman et al. 1999; North, 2002). Recently, it was shown that selective insertion of organelle‐bound receptors into the plasma membrane upon organelle exocytosis facilitates cellular secretion (Miklavc et al. 2011a, 2013; Thompson et al. 2013). In addition, it was also suggested that P2X4 receptors exercise a physiological role within the lysosomal compartment facilitating endolysosomal membrane fusion upon alkalinization and activation of the receptor (Huang et al. 2014; Cao et al. 2015).
In the present study, we provide evidence that internalization of P2X4 receptors and trafficking to acidic compartments facilitate re‐sensitization of P2X4 receptors. In particular, re‐sensitization depends on a protonation/de‐protonation cycle of critical histidine residues within the extracellular loop of P2X4 receptors that is mediated by receptor internalization and recycling. Interestingly, re‐sensitization is completely blocked in the presence of receptor agonist ATP. These data provide further evidence for the physiological importance of P2X4 residing in acidic compartments and, based on observations from other receptor subtypes, it might well constitute a more general mechanism for receptor re‐sensitization.
Methods
Preparation of buffers and experimental solutions
All materials were purchased from Sigma (Darmstadt, Germany) unless otherwise stated. Ambroxol [trans‐4‐(2‐amino‐3,5‐dibromobenzylamino)‐cyclohexanole] hydrochloride) was obtained from Boehringer Ingelheim (Biberach, Germany). All cell culture media were obtained from Gibco (ThermoFisher, Darmstadt, Germany).
Experimental solutions
Buffers contained (in mm): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 glucose and 10 Hepes and the pH was adjusted to the respective values. For solutions containing ATP, the pH was adjusted after addition of ATP.
Cells were incubated with 100 nm bafilomycin A1, 100 μm ambroxol, 80 μm dynasore, 5 mm NEM or 10 μg mL–1 brefeldin A for 30 min before the start of the experiments, as well as for the duration of the experiment.
Cell culture
The TsA201 cell line is a derivative of the human embryonic kidney cell line HEK‐293 (ATCC#CRL1537) that expresses a T‐antigen against the simian virus 40. TsA cells were maintained in minimal essential medium supplemented with 50 U mL–1 penicillin, 50 μg mL–1 streptomycin, 2 mm l‐glutamine (Boehringer, Mannheim, Germany) and 10% fetal calf serum (Gibco) at 37°C in a humidified atmosphere at 95% air and 5% CO2. For patch clamp measurements, cells were grown on polyornithine‐coated culture dishes to 40% confluence, infected with adenoviruses encoding P2X4‐EGFP (wt) and used for experimentation 24–48 h after transfection.
HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 50 U mL–1 penicillin, 50 μg mL–1 streptomycin, 2 mm l‐glutamine (Boehringer) and 10% fetal calf serum (Gibco). For fura‐2 measurements, HeLa cells were grown in six‐well plates, infected with adenoviruses encoding P2X4‐EGFP (wt) and, if applicable, transfected with plasmids encoding dnm2(K44A). After 24 h, infected (transfected) cells were seeded into microchannel slides (μ‐slide; IBIDI, Munich, Germany) and maintained in DMEM supplemented with 50 U mL–1 penicillin, 50 μg mL–1 streptomycin, 2 mm l‐glutamine (Boehringer) and 10% fetal calf serum (Gibco) for 24 h before start of the experiment.
Electrophysiology
Whole‐cell patch clamp recordings were performed as described previously (Ludolph et al. 2010). Briefly, TsA201 cells were used for experiments 24 h after transfection. Membrane currents were recorded in the whole‐cell recording mode using an EPC‐9 amplifier and Patchmaster, version 2x73 (HEKA, Lambrecht, Germany). Before recording, cells were rinsed twice with an extracellular standard solution containing (in mm): 145 NaCl, 5 KCl, 1.5 CaCl2, 10 glucose and 6 Hepes [4‐(2‐hydroxyethyl)piperazine‐1‐ethanesulphonic acid, N‐(2‐hydroxyethyl)piperazine‐N′‐(2‐ethanesulphonic acid), 6 Mes (2‐[N‐morpholino]ethansulphonic acid); pH 7.4]. Patch pipettes were drawn from borosilicate glass with tip resistances of ∼2 MΩ when filled with (in mm): 125 CsF, 10 NaF, 10 EGTA [ethylene glycol‐bis(2‐aminoethylether)‐N,N,N′,N′‐tetraacetic acid], 10 Hepes; pH 7.2. To improve sealing, tips were briefly dipped into 2% dimethylsilane dissolved in dichloromethane. Unless otherwise stated, cells were held at a holding potential of –80 mV from which channel activation was elicited by fast agonist application.
Perforate measurements using amphotericin B were essentially performed as described previously (Linely, 2013). Briefly, the tip of the capillary was filled with an internal solution composed of in (mm): 100 cesium methanesulphonate, 10 NaCl, 1 MgCl2, 10 EGTA and 40 Hepes, pH 7.3. For backfilling, amphotericin B (stock: 40 mg mL–1 in dimethylsulphoxide) was added to this solution to achieve a final concentration of 400 μg mL–1. A stable access resistance around 20 MΩ was typically achieved within 5 min. To control for perforated conditions throughout the experiment (i) uncompensated and compensated capacitive current transients were recorded upon a small voltage jump immediately before and after the experimental recording to analyse the serial resistance and (ii) Na‐fluorescein (1 mm; MW 376) was included in the pipette solution. Changes in capacitive transients (resembling transients routinely observed at whole‐cell recordings) in the course of the experiment correlated with the onset of Na‐fluorescein influx into the cells. These cells were omitted from analysis. Valid experiments had stable recordings with almost unchanged access resistance (∼20 MΩ) before and after the experiment and yielded no fluorescence signal from the cells.
The medium in the dish (1.5 mL) was continuously exchanged using a ‘global’ bath perfusion with the inflow set to 4.5 mL min–1 and the outflow removing any excess fluid. Reagents were applied locally to the cells by the L/M‐SPS‐8 superfusion system (List, Darmstadt, Germany). Switching between the eight channels of the superfusion system was controlled by magnetic valves. The local inlet (tip of an eight‐barrelled pipette) was positioned at a distance of 50–100 μm upstream and the local outlet at ∼300 μm downstream of the patch pipette. A constant flow rate of control and test solutions (1 mL min–1) was achieved by means of a pressure control system (MPCU‐3; Lorenz, Göttingen, Germany). The time of solution exchange was estimated from the changes in the liquid junction potential to be ∼1 ms.
Fura‐2 measurements
For measurement of changes in intracellular calcium levels, HeLa cells were loaded with 3 μm fura‐2 AM (ThermoScientific, Karlsruhe, Germany) for 20 min in DMEM, washed twice in bath solution (in mm: 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5 glucose and 10 Hepes; pH 7.4) and maintained in bath solution till the start of the experiments. Using a 2‐D imaging system, cells were illuminated for 50 ms at a rate of 1 Hz at 340 and 380 nm (Haller et al. 1999) and images were acquired using MetaFluor (Molecular Devices, Ismaning, Germany). Images were analysed using MetaFluor Analyst (Molecular Devices) as described previously (Miklavc et al. 2011b). A rapid perfusion exchange system was used to switch between different experimental solutions.
P2X4‐phluorin receptor distribution
The contribution of fluorescence from P2X4‐phluorin123 receptors on the cell surface, within acidic compartments and within other intracellular compartments was calculated as described peviously (Xu et al. 2014). In brief, these experiments exploit the pH sensitivity of pHluorin, which is non‐fluorescent under acidic conditions. The cells were initially bathed in extracellular solutions at pH 7.4. Under these settings, the total cellular fluorescence (F total) consists of channels on the surface (F surface) with the pHluorin facing the extracellular pH 7.4 buffer, channels within acidic compartments (F acidic) with the pHluorin quenched and receptors/fluorescence in other compartments (F other) of unknown pH environment. Hence, F total = F surface + F acidic + F other.
Fluorescence in individual compartments was calculated as: F surface = F basal − F other; F acidic = F total − F basal; F other = F total − F acidic − F surface. F basal is the basal fluorescence of the cells at pH 7.4 and F Total is the fluorescence of the cells in NH4Cl, when all acidic compartments are also at a pH greater than 7.4. F surface was measured by applying extracellular buffers at pH 5.4, which quenches surface receptors. F acidic was measured by applying NH4Cl, which alkalizes acidic compartments to a pH of 7.4 (Miesenbock et al. 1998).
P2X4‐EGFP internalization
To analyse the internalization of (wt)P2X4 and P2X4(H286A) upon stimulation with ATP, Hela cells were transfected with either (wt)P2X4‐EGFP or P2X4(H286A)‐EGFP, respectively. Quantitative analysis of receptor internalization was performed as described previously (Stauffer et al. 1998). In brief, a time series of confocal fluorescence images of cells expressing either P2X4‐EGFP probe was recorded before and after receptor stimulation. The relative change in the plasma membrane fluorescence intensity was determined in each image using line intensity profiles across each of the cells (Fig. 5 F). The relative decrease in the fluorescence intensity of the plasma membrane to that of the cytosol was calculated by measuring the amplitude of the fluorescence signal at the plasma membrane and dividing it by an average intracellular fluorescence intensity (F PM/F Cyt) (Fig. 5 F)
Figure 5. P2X4 re‐sensitization is reduced in the presence of its ligand ATP.
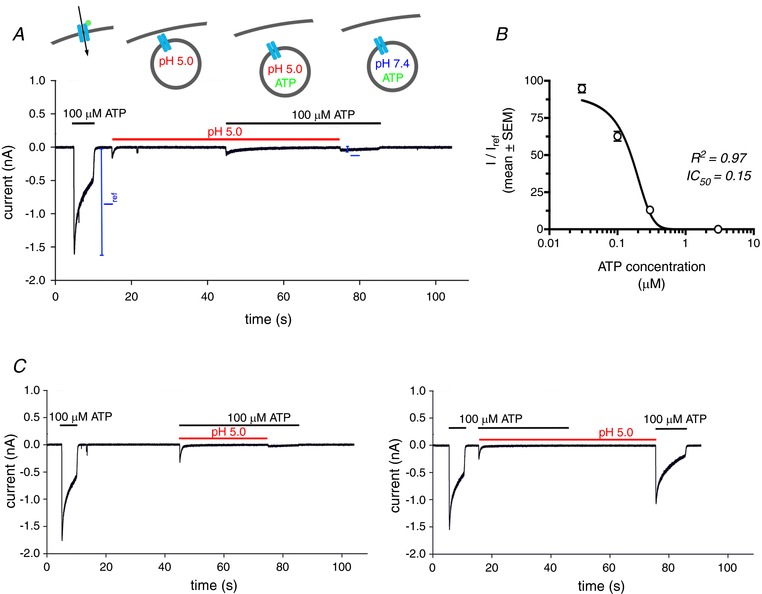
A, whole‐cell patch clamp recording from an individual TsA cell expressing (wt)P2X4‐EGFP. Lines indicate the presence of ATP (black) and/or low pH (red) in the extracellular solution. The depicted experiment mimics activation (100 μm ATP, pH 7.4), internalization/acidification (pH 5.0), residence in acidic compartment containing ATP (100 μm ATP, pH5) and subsequent activation following alkalinization (100 μm ATP, pH 7.4) cycle as depicted in the schematic drawings above the recording. B, normalized receptor currents from 3–6 individual cells depicting the ratio between the peak current following the switch to 100 μm ATP, pH 7.4 as depicted in (A) (I) and the peak current following the initial ATP application (I ref). The inhibitory effect of ATP under acidic conditions had an IC50 = 0.15 μm. C, whole‐cell patch clamp recordings from TsA cells expressing (wt)P2X4‐EGFP. The ATP‐dependent inhibition of P2X4 re‐sensitization (left) could be fully abrogated by brief acidification of the receptor in the absence of ATP (right).
Plasmids and adenoviruses
Adenoviruses encoding rat P2X4‐EGFP (wt) were described recently (Miklavc et al. 2011a). Original constructs were a kind gift from R. D. Murrell‐Lagnado (University of Cambridge, Cambridge, UK). Adenoviruses encoding dnm2(K44A) were received form S. Schmid (UT Southwestern, Dallas, TX, USA).
Site‐directed mutations in the P2X4‐EGFP plasmids were generated by Gibson assembly using the In‐Fusion HD Cloning Kit (Clontech, Palo Alto, CA, USA). In short, primers containing the respective mutation sequence and a 15 bp overlap with each other at their 5′ ends were designed (Biomers, Ulm, Germany) to run the polymerase chain reaction (PCR) reaction. Linearized PCR products were transformed into Stellar™ Competent Cells (Takara Bio Inc., Otsu, Japan) for ligation. All mutations were confirmed by sequencing (GATC Biotech, Konstanz, Germany). Generation and characterization of P2X4‐pHluorin is described in (Khakh & North, 2006; Xu et al. 2014).
Statistical analysis
All statistics and the construction of graphs were performed in Excel (Microsoft Corp., Redmond, WA, USA) and Prism 5 (GraphPad, San Diego, CA, USA). The data are reported as the mean ± SEM, unless stated otherwise. Statistical significance was determined using analysis of variance (ANOVA with Bonferroni's correction) for multiple comparisons and an unpaired, two‐tailed Student´s t test for comparison of two independent samples. P < 0.05 was considered statistically significant.
Results
P2X4 receptor is re‐sensitized by acidic pH
Two forms of desensitization have been described for P2X4 receptors: (i) a decline in membrane current is observed during the continued application of ATP and (ii) a decline in the peak current with repeated brief applications of ATP (‘run‐down’). This run‐down was only observed in whole‐cell but not perforated patch recordings. It has been postulated that diffusible cytosolic factors regulating the receptors are lost during intracellular dialysis (Fountain & North, 2006; Zemkova et al. 2015). Yet, so far, no such factor has been identified in any substitution experiments. Performing whole‐cell patch clamp experiments in TsA cells expressing (wt)P2X4‐EGFP, we found that run‐down of receptor currents upon repeated ATP application could be fully abrogated by brief perfusion (‘priming’) of cells with acidic bath solution (Fig. 1 A). Interestingly, compared to the initial ATP evoked response, currents were often increased following the pH‐wash. Further characterization of the pH‐dependent re‐sensitization revealed that (extracellular) acidification for 30–60 s is sufficient for full receptor re‐sensitization. When cells were perfused (‘primed’) with acidic solution (pH 5.0) for various shorter periods, partial recovery of the ATP‐induced current could be observed, whereas a subsequent 1 min perfusion with acidic solution resulted in full re‐sensitization (Fig. 1 B).
Figure 1. P2X4 receptor current run‐down is reversed by acidic pH.
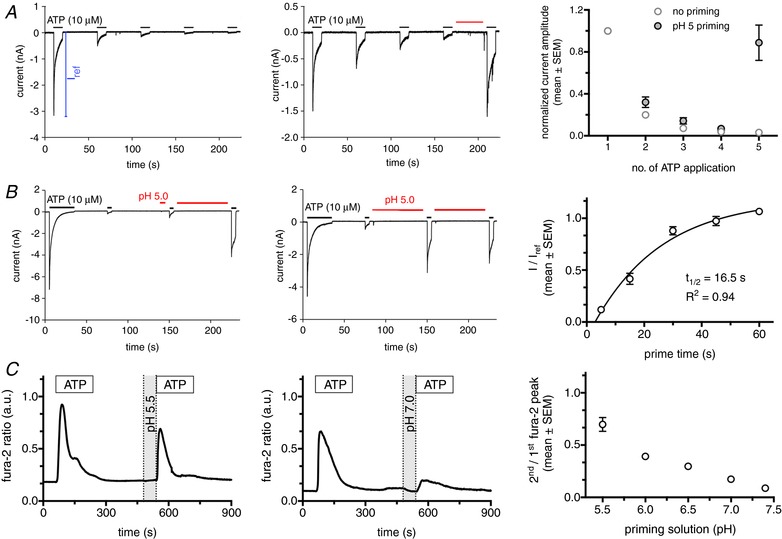
A, left: whole‐cell patch clamp recordings from a TsA cell expressing (wt)P2X4‐EGFP illustrating receptor current run‐down upon repeated ATP application. Middle: run down of receptor currents upon repeated ATP application was fully abrogated by brief perfusion of cells with acidic bath solution (indicated by red bar). Right: normalized receptor currents from five individual cells following repetitive ATP application (every min) in the absence (no priming) or presence (pH 5 priming) of a 30 s wash with acidic bath solution before the final ATP application. Respective currents were normalized to the peak current measured in any single cell following the initial ATP application (I ref, as illustrated in A). B, left, middle: whole‐cell patch clamp recordings from individual TsA cells expressing (wt)P2X4‐EGFP with varying application times of priming solution (pH 5). Right: normalized receptor currents from eight individual cells following varying priming periods (pH 5). Current amplitudes following the first pH wash (I) were normalized to the peak current following the initial ATP application (I ref). C, left, middle: fura‐2 ratio traces from individual HeLa cells expressing (wt)P2X4‐EGFP. Cells were stimulated with 100 μm ATP for 3 min, followed by a recovery period of 4 min before perfusion with priming solution (1 min) of varying pH and before repeated ATP application. Right: graph depicting ratios of second/first Ca2+ peak following repetitive ATP application (5 min apart) in dependence on the pH of the priming solution before the second ATP application (n > 77 cells for each condition).
In addition to patch clamp experiments, we also performed intracellular Ca2+ ([Ca2+]i) measurements to characterize receptor re‐sensitization in intact cells preventing loss of diffusible, cytosolic factors. Accordingly, we expressed (wt)P2X4‐EGFP in HeLa cells. Stimulation of HeLa cells with 100 μm UTP resulted in a small increase in [Ca2+]i that was abolished by suramin treatment, a selective P2Y receptor antagonist, indicating low expression of P2Y receptors. Therefore, all subsequent experiments were performed in the presence of suramin (800 μm) to inhibit P2Y induced increases in [Ca2+]i (Thompson et al. 2013). Activation of P2X4 receptors results in strong influx of Ca2+ in HeLa cells transfected with (wt)P2X4‐EGFP (Fig. 1 C). Suramin treatment did not affect P2X4 induced [Ca2+]i signals. To further characterize the effect of acidification on receptor re‐sensitization, we stimulated HeLa cells expressing (wt)P2X4‐EGFP with 100 μm ATP (3 min) to achieve full desensitization of surface P2X4 receptors. Following 4 min of perfusion with bath solution (to allow complete return of intracellular Ca2+ levels to baseline) and a 1 min perfusion with priming (acidification) solutions, cells were again stimulated with 100 μm ATP (3 min). Analysing the ratio of the [Ca2+]i signals revealed that the re‐sensitization of P2X4 receptors was dependent on the degree of acidification. At pH 7.4, the response to the second ATP application was less than 10% of the first response (8.8 ± 0.02; n = 125). This value gradually increased with lower pH values to ∼70% (69.7 ± 0.07; n = 77) at pH 5.5 (Fig. 1 C). Lowering pH even further had no additional effect.
These observations suggest that acidification of the extracellular loop of the receptor is required for re‐sensitization.
P2X4 receptor re‐sensitization depends on recycling via acidic organelles
One possible physiological mechanism for acidification of the extracellular loop is internalization and recycling of the receptor. The luminal pH of endocytic organelles is acidic, and acidification and its regulation constitute an important part of endosome maturation (Huotari & Helenius, 2011; Mindell, 2012).
P2X4 receptors undergo rapid and constitutive internalization and subsequent recycling back to the plasma membrane (Bobanovic et al. 2002). Moreover, trafficking is increased following receptor stimulation with ATP (Royle et al. 2002). In such a scenario, surface receptor re‐sensitization should depend on the time required for receptor recycling (re‐sensitization will increase as more receptors are being recycled). To test this hypothesis, we increased the interval between applications of ATP. These experiments revealed that re‐sensitization is almost complete following a 30 min recovery time. The Ca2+‐peak following the second ATP application is 89.6 ± 32.3 of initial Ca2+‐peak (n = 74) (Fig. 2), in line with previously observed time courses for recycling (Bobanovic et al. 2002). To further substantiate the hypothesis that trafficking and recycling of the receptors via acidic compartments is required for receptor re‐sensitization, we performed experiments inhibiting either organelle acidification [100 nm bafilomycin A1 (Yoshimori et al. 1991), 100 μm ambroxol (Fois et al. 2015)], clathrin‐mediated endocytosis [overexpression of the dynamin2 K44A mutant (Damke et al. 2001), treatment with 80 μm dynasore (Macia et al. 2006)] or exocytosis to prevent re‐insertion of internalized P2X4 (5 mm NEM) (Rodriguez et al. 1994) (Fig. 3 A). All of these perturbations significantly reduced the Ca2+‐peak following a second ATP application 30 min after the first ATP application (<19% for all conditions) (Fig. 3 B). By contrast, inhibition of the secretory pathway with 10 μg mL–1 brefeldin A to inhibit delivery of newly synthesized P2X4 receptors to the plasma membrane (Klausner et al. 1992) did not significantly inhibit receptor re‐sensitization (Fig. 3 B). This supports our hypothesis that receptor recycling and not synthesis is the main factor responsible for recovery of P2X4 activation.
Figure 2. P2X4 receptor re‐sensitization is time‐dependent.
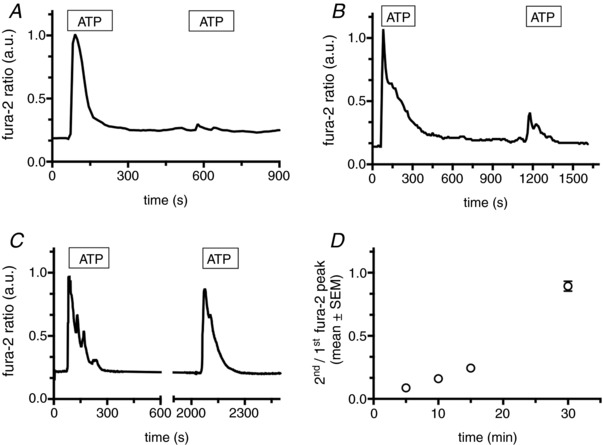
A–C, fura‐2 ratio traces from individual HeLa cells expressing (wt)P2X4‐EGFP. Cells were stimulated with 100 μm ATP for 3 min, followed by recovery periods of 5 min (A), 10 min (B) and 30 min (C), respectively. D, ratios of second/first Ca2+ peak following repetitive ATP application in dependence on the lag between first and second ATP application (n > 62 cells for each condition).
Figure 3. P2X4 receptor re‐sensitization depends on receptor recycling via acidic organelles.
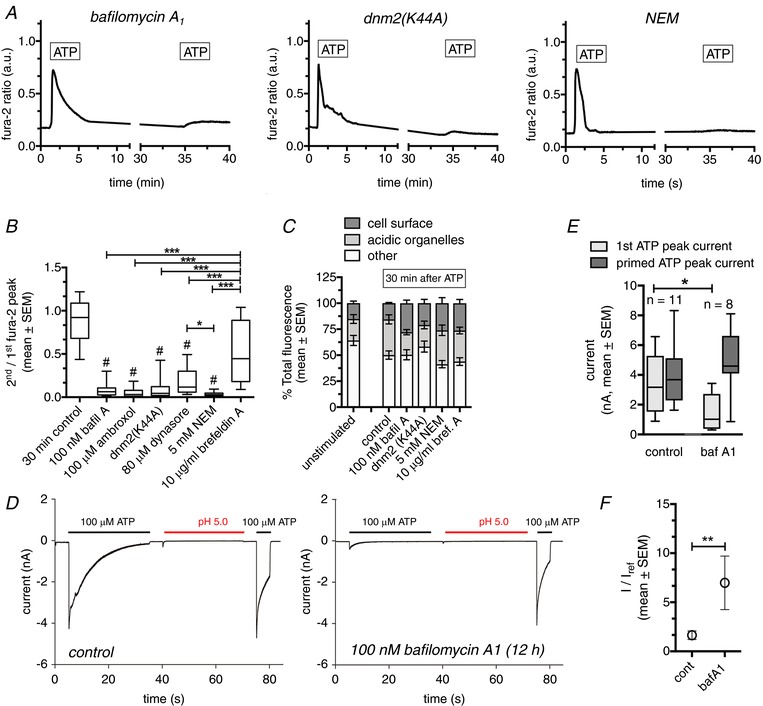
A, fura‐2 ratio traces from individual HeLa cells expressing (wt)P2X4‐EGFP. Cells were stimulated with 100 μm ATP for 3 min, followed by recovery periods of 30 min before the second application of ATP. Cells were treated with inhibitors of organelle acidification (100 nm bafilomycin A1), clathrin‐mediated endocytosis (overexpression of the dynamin2 K44A mutant) or exocytosis (5 mm NEM). B, ratios of second/first Ca2+ peak following repetitive ATP application (30 min apart) in the absence (30 min control) or the presence of various inhibitors of the organelle acidification, internalization, recycling or the secretory pathway. n = 41–135 cells per condition. #Statistical significant difference to control, *Statistical significant difference between respective treatments. C, proportions of P2X4‐pHluorin receptors within different cellular compartments of unstimulated Hela cells (baseline) or 30 min following stimulation of the cells with 100 μm ATP in the absence (control) or the presence of various inhibitors of the organelle acidification, internalization, recycling or the secretory pathway. n = 10–27 cells per condition. D, whole‐cell patch clamp recordings from TsA cells expressing (wt)P2X4‐EGFP under control conditions (left), or in cells treated with 100 nm bafilomycin A1 for 12 h (right). E, quantitative analysis of the peak currents following the initial ATP application (light grey) and the ATP application following priming (pH 5, dark grey) in TsA cells expressing (wt)P2X4‐EGFP under control conditions, or in cells treated with 100 nm bafilomycin A1 for 12 h. F, normalized P2X4 receptor currents following priming with pH 5 from TsA cells expressing (wt)P2X4‐EGFP under control conditions or in cells treated with 100 nm bafilomycin A1 for 12 h. Current amplitudes following the initial pH wash (I) were normalized to the peak current following the initial ATP application (I ref). Data are from 8–11 cells for each condition.
To exclude the possibility that the reduced response to the second application of ATP is a result of a reduction in the number of cell surface receptors under different experimental conditions, we evaluated the distribution of receptors at the cell surface, within acidic compartments and within other intracellular compartments using a pH‐sensitive, fluorescence‐tagged P2X4 receptor (P2X4‐pHluorin123) (Xu et al. 2014). In unstimulated cells (baseline), 15.2 ± 2.0% of total P2X4 receptors were expressed at the cell surface (Fig. 3 C). This is in line with the result of a previous study (Xu et al. 2014) and the notion that P2X4 receptors are primarily localized in intracellular compartments (Robinson & Murrell‐Lagnado, 2013). We then analysed changes in P2X4 surface receptor expression 30 min after application of ATP under various experimental conditions. Treatment with bafilomycin A1 increased total fluorescence by an average of 70.1%, indicating that bafilomycin A1 treatment removed the acidification of the organelles. None of the treatments resulted in a significant reduction in the percentage of P2X4 receptors at the cell surface. Under control conditions (no interference with receptor internalization/recycling), ATP stimulation resulted in a significant increase in P2X4 receptors within acidic organelles compared to baseline (34.3 ± 4.3%, n = 25 vs. 20.6 ± 4.2%, n = 27, P = 0.04), respectively. This is consistent with previous studies reporting that internalization and recycling is increased following P2X4 receptor stimulation (Royle et al. 2002) (Fig. 3 C). In summary, these data suggest that the observed reduction of the Ca2+‐peak following a second ATP stimulus is a result of impaired recycling via acidic organelles, and not a result of reduced P2X4 expression on the cell surface.
Moreover, incubation of TsA cells expressing (wt)P2X4‐EGFP with 100 nm bafilomycin A1 overnight, to inhibit acidification of recycling organelles, resulted in significantly reduced currents following an initial stimulation with 100 μm ATP compared to untreated control cells (1.49 ± 0.45, n = 8 vs. 3.26 ± 0.62, n = 11, P = 0.047, respectively). However, subsequent priming with acidic solution (pH 5.0, 30 s) fully re‐established ATP‐induced currents to levels observed in untreated control cells (4.84 ± 0.77, n = 8 vs. 4.00 ± 0.69, n = 11, P = 0.43, respectively) and resulted in significantly increased priming because of the decreased I ref (Fig. 3 D–F). These data further indicate that activation of P2X4 receptors recycled via acidic organelles significantly contributes to the ATP induced current and that inhibition of recycling and/or acidification inhibits P2X4 re‐sensitization.
P2X4 re‐sensitization depends on a protonation/de‐protonation of histidines in the extracellular loop
We next aimed to identify the molecular cause for this pH‐dependent re‐sensitization. Several amino acid residues have been found to mediate pH‐dependent modulation of pH‐sensitive ion channels and receptors (Liu et al. 2009). In P2X receptors, histidines in the extracellular loop have been found to modulate receptor function in a pH‐dependent manner (North, 2002; Liu et al. 2009; Kaczmarek‐Hajek et al. 2012). We therefore generated and expressed P2X4 mutants where all histidines (H140, H241 and H286) in the extracellular loop (i.e. inside the lysosomal compartment following internalization) (Fountain et al. 2007; Huang et al. 2014) were replaced by either alanine (A, to inhibit protonation), arginine (R, protonation mimetic) or aspartate (D, deprotonation mimetic). Initial experiments revealed that complete exchange of histidines (triple mutants) significantly attenuated the pH‐dependent re‐sensitization of P2X4 receptors (Fig. 4 A), with stronger effects for the alanine and arginine substitutions. Screening individual histidine substitutions using the Ca2+‐peak assay, revealed that replacing histidines with protonation mimetics (R) significantly reduced pH‐dependent re‐sensitization (2nd/1st fura‐2 peak), irrespective of the histidine position. Moreover, these experiments identified stronger effects at residues H241 and residue H286. Mutants H286A and H286R were the most effective, with H286R resulting in almost complete inhibition of pH‐dependent re‐sensitization (Table 1).
Figure 4. P2X4 re‐sensitization depends on a protonation/de‐protonation of histidines in the extracellular loop.
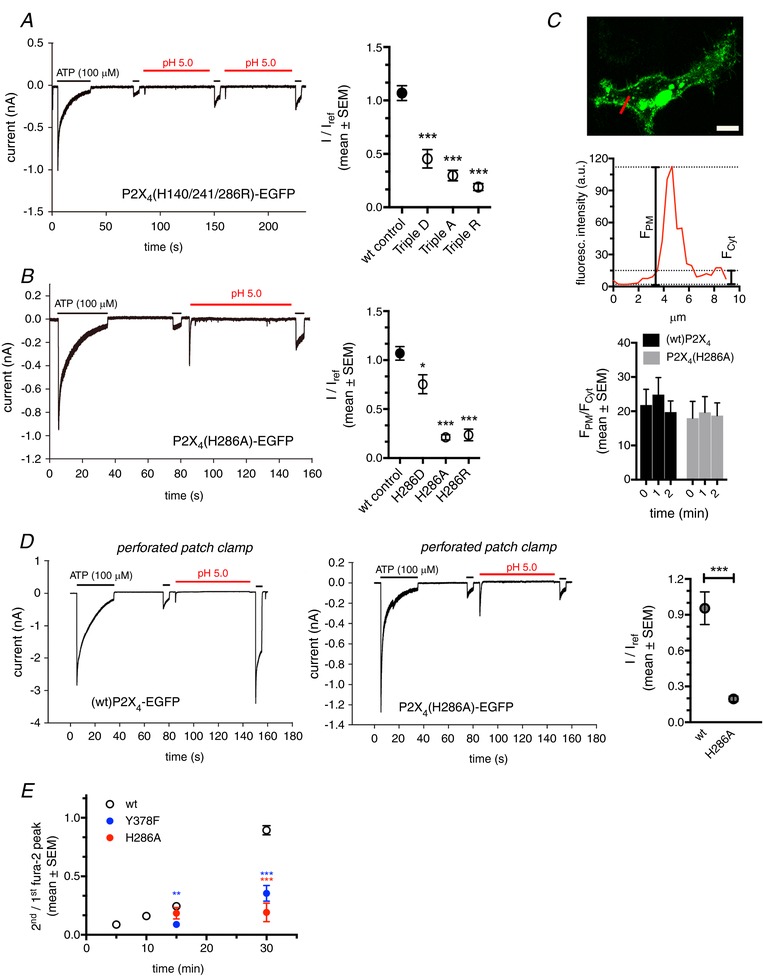
A, left: whole‐cell patch clamp recording from a TsA cell expressing TripleR P2X4‐EGFP. Right: normalized P2X4 receptor currents following priming with pH 5 from TsA cells expressing (wt)P2X4‐EGFP (wt control) or exchange of extracellular histidines to either aspartate (Triple D), alanine (Triple A) or arginine (Triple R). Current amplitudes following the initial pH wash (I) were normalized to the peak current following the initial ATP application (I ref). Data are from ≥10 cells for each conditions. ***Statistical significance compared to wild‐type control (ANOVA). B, left: whole‐cell patch clamp recording from individual a TsA cell expressing P2X4(H286A)‐EGFP. Right: normalized P2X4 receptor currents following priming with pH 5 from TsA cells expressing (wt)P2X4‐EGFP (wt control) or mutations of H286. ATP‐induced current amplitudes following the initial pH wash (I) were normalized to the peak current following the initial ATP application (I ref). Data are from 5–12 cells for each conditions. *Statistical significance compared to wild‐type control (ANOVA). C, top: confocal image of Hela cell expressing (wt)P2X4‐EGFP. The fluorescence intensities along the red line were plotted as line intensity histogram, shown in the middle. Calculation of F PM/F Cyt was used to quantify the extent of membrane localization. Bottom: F PM/F Cyt before (0 min) and shortly after (1 and 2 min) stimulation with ATP for cells expressing (wt)P2X4‐EGFP or P2X4(H286A)‐EGFP. Data are from >10 cells for each conditions. Scale bar = 10 μm. D, left: perforated patch clamp recordings from TsA cells expressing (wt)P2X4‐EGFP. Middle: perforated patch clamp recordings from TsA cells expressing P2X4(H286A)‐EGFP. Right: normalized P2X4 receptor currents from TsA cells expressing (wt)P2X4‐EGFP or P2X4(H286A)‐EGFP following priming with pH 5.0. ATP‐induced current amplitudes following the pH wash (I) were normalized to the peak current following the initial ATP application (I ref). Data are from 5–6 cells for each condition. *Statistical significance compared to wild‐type control. E, ratios of second/first Ca2+ peak following repetitive ATP application at different recovery times for wt, internalization (Y378F)‐ and protonation (H286A)‐deficient P2X4. (n > 14 cells for each condition). *Statistical significance when compared to wild‐type control at individual times of second ATP application.
Table 1.
Ratio of second/first ATP‐induced Ca2+ peak (2nd/1st fura‐2 peak) (Fig. 1 C) in cells expressing wild‐type or indicated mutants of P2X4
| P2X4 mutant | 2nd/1st fura‐2 peak | N | P value |
|---|---|---|---|
| Wild‐type | 0.57 ± 0.06 | 102 | |
| H140D | 0.34 ± 0.09 | 25 | 0.11 |
| H140A | 0.40 ± 0.06 | 40 | 0.22 |
| H140R | 0.31 ± 0.07 | 34 | 0.02 |
| H241D | 0.38 ± 0.06 | 55 | 0.05 |
| H241A | 0.34 ± 0.05 | 57 | 0.01 |
| H241R | 0.25 ± 0.07 | 30 | 0.004 |
| H286D | 0.45 ± 0.10 | 25 | 0.76 |
| H286A | 0.20 ± 0.03 | 65 | <0,0001 |
| H286R | 0.05 ± 0.02 | 36 | <0,0001 |
Cells were primed by a brief (1 min) perfusion with acidic (pH 5) solutuion before the second ATP application. P values are derived from ANOVA.
These data were confirmed in patch clamp experiments (Fig. 4 B). The reduced effect of the H286D substitution on priming (I/I ref) is probably the result of a strongly reduced initial (I ref) current because the absolute current (I) following priming is in a range similar to that for the H286A and H286R mutants. Peak currents (I ref) for P2X4(H286A)‐EGFP, P2X4(H286R)‐EGFP and P2X4(H286D)‐EGFP were reduced by 56.3%, 11.0% and 97.7%, respectively, compared to (wt)P2X4‐EGFP, even though we had already used four‐fold increased DNA for transfection as described previously (Clarke et al. 2000). Again, we can exclude an effect of differential trafficking (i.e. internalization of surface receptors) as a cause for the observed reduction in the second Ca2+ peak or current, respectively. First of all, the interval between I ref and the current induced by the ATP application following priming (I) is less than 2 min and probably too short to induce major differences in surface expression. Second, we performed experiments analysing the ratio between EGFP fluorescence intensity on the cell surface and the intracellular region at various time points following ATP addition. We did not detect a significant difference in the ratios for (wt)P2X4‐EGFP and P2X4(H286A)‐EGFP within 2 min after ATP addition (Fig. 4 C).
We also preformed experiments in the perforated patch clamp configuration to investigate the importance of H286 protonation under conditions without ‘run‐down’ effects observed in the whole cell configuration. Similar to whole cell experiments, our standard protocol (100 μm ATP for 30 s) resulted in almost complete de‐sensitization of P2X4 receptor currents in cells expressing either (wt)P2X4‐EGFP or P2X4(H286A)‐EGFP. An immediate, additional ATP application after 30 s (to restrict the effect of receptor recycling) yielded strongly reduced currents. Subsequent priming with acidic solution (pH 5.0 for 60 s) resulted in a clear proton‐mediated re‐sensitization for wild‐type receptors and a strongly reduced re‐sensitization for the H286A mutant. These measurements confirmed our findings from the whole‐cell recordings (Fig. 4 D).
Overall, these data again indicate that protonation is critical for re‐sensitization similar to the bafilomycin A1 experiments (Fig. 3). In summary, our observation that all three mutants (D, A, and R substitutions) exerted significant effects on the priming capacity of P2X4 suggests that protonation as well as de‐protonation of critical histidine residues is crucial for full recovery of receptor availability (see model in Fig. 6).
Figure 6. Proposed model for P2X4 re‐sensitization.
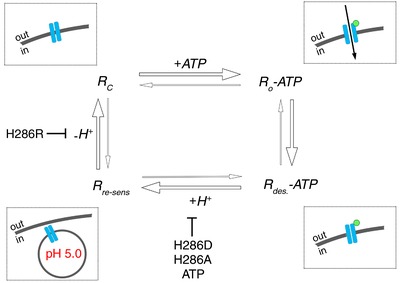
Closed P2X4 receptors (Rc) are expressed in the plasma membrane (insert). Addition of extracellular ATP (insert, green circle) results in opening of the closed receptor (Ro‐ATP) and subsequent desensitization (Rdes‐ATP). Desensitized receptors are internalized and the acidic environment within the endo‐/lysosomal organelles (insert) results in receptor re‐sensitization (Rre‐sens). Subsequent alkalinization of the receptor environment is essential to return the receptor to its active, closed conformation (Rc). P2X4 re‐sensitization depends on protonation and de‐protonation of critical histidine residues in the extracellular loop and is perturbed by mutations that inhibit either protonation or de‐protonation. Also, the P2X4 ligand ATP inhibits P2X4 re‐sensitization when present under acidic conditions.
To further substantiate our finding that receptor recovery is accelerated by recycling and protonation within acidic organelles, we analysed the recovery from desensitization for an internalization‐deficient (Y378F) and a protonation‐deficient (H286A) P2X4 mutant at pH 7.4. Recovery from de‐sensitization is significantly slower for both constructs. After 30 min at pH 7.4, the Ca2+‐peak following the second ATP application is 19.1 ± 7.9% (n = 15) and 35.5 ± 6.8% (n = 25) of the initial Ca2+‐peak for P2X4(H286A)‐EGFP and P2X4(Y378F)‐EGFP, respectively. This is significantly less than the 89.6 ± 32.3% recovery observed for (wt)P2X4‐EGFP of initial Ca2+‐peak (Fig. 4 E). Even after 1 h, recovery was only 67.6 ± 8.1% (n = 24) for P2X4(Y378F)‐EGFP. The observed recovery is probably overestimated because we frequently found reduced initial signals in cells expressing these mutants. This potentially resulted from receptors that had already been exposed to ATP at some time during the cell culture and could not recover because of internalization/protonation deficiency. The reduced initial signal then results in ‘increased’ priming effects.
Protonation‐dependent P2X4 re‐sensitization is reduced in the presence of its ligand ATP
P2X4 receptors are primarily localized on lysosomal organelles where these receptors are in an inactive state because of the acidic environment. Recently, it was shown that P2X4 receptors and its ligand ATP are enriched in lysosomes and that increasing the luminal pH in these organelles caused P2X4 activation (Huang et al. 2014). To mimic such a scenario and to test whether re‐sensitization is affected by the presence of the agonist, we performed experiments mimicking an activation (100 μm ATP, pH 7.4), internalization/acidification (0 mm ATP, pH 5), residence in acidic compartments containing ATP (1 mm ATP, pH 5) and subsequent activation following alkalinization (or exocytosis, 100 μm ATP, pH 7.4) cycle (Fig. 5 A). Re‐sensitization of P2X4 receptors was almost completely inhibited when ATP was present during acidification (Fig. 5 A). This inhibitory effect of the agonist ATP under acidic conditions was dose‐dependent. The inhibitory effect was only lost at very low concentrations of ATP (IC50 = 0.15 μm) (Fig. 5 B). This effect was not dependent on prior acidification of the receptor (Fig. 5 C); instead, it could be fully abrogated by brief acidification of the receptor in the absence of ATP (similar to the re‐sensitization observed in the previous study) (Fig. 5 D). These data suggest that, next to the observed protonation/de‐protonation of P2X4, additional, yet unidentified, mechanisms contribute to full re‐sensitization of receptor function and that P2X4 is probably shielded from ATP when present in acidic organelles.
Discussion
P2X receptors are widely expressed and mainly act as cation‐permeable, ligand‐gated ion channels at the plasma membrane of most cells. Yet, some, in particular P2X4 receptors, are predominately found on the limiting membrane of intracellular organelles, specifically lysosomes or lysosome‐related organelles, which are characterized by their acidic lumen. The physiological role of this stable expression in acidic compartments has been a long‐standing puzzle because the acidic pH inside these organelles inhibits receptor activation (North, 2002). Recently, it was shown that selective insertion of organelle‐bound receptors into the plasma membrane facilitates cellular secretion (Miklavc et al. 2013). It has also been suggested that P2X4 receptors exercise a physiological role within the lysosomal compartment, facilitating endolysosomal membrane fusion upon alkalinization and activation of the receptor (Huang et al. 2014; Cao et al. 2015). In the present study, we now provide evidence that targeting of P2X4 receptors to acidic organelles is essential for receptor re‐sensitization. Similar observations (trafficking‐dependent re‐sensitization) have been made for other ion channels and receptors, including other P2X receptors.
However, the molecular mechanisms, such as how internalization and recycling of P2X receptors affects re‐sensitization, remain elusive. Our findings indicate that the internalization/recycling via acidic organelles facilitates protonation/de‐protonation of critical histidine residues within the extracellular loop as required for receptor re‐sensitization, in particular H286. The most simplistic model to explain and converge the effects of H286 on the pH‐sensitivity of receptor activation and recovery from desensitization would be that protonation of H286 under acidic conditions displaces ATP from its binding site. Many surface receptors undergo endocytosis to deposit their ligands within the cell and are recycled back to the plasma membrane for further uptake of ligands. The acidic pH within late endosomes thereby facilitates the dissociation of the ligand from the receptor (Yamashiro & Maxfield, 1984). It is quite conceivable that internalization of P2X4 receptors following ATP binding and activation exploits the same mechanism to facilitate dissociation of ATP from the desensitized receptor and transform the receptor back to its closed, activatable from. Subsequent recycling to the cell surface is then the final step required for repeated P2X4 activation. Therefore, de‐sensitization of P2X4 receptors in the ATP bound state regulates the kinetics of signal transduction, whereas recycling regulates the cellular responsiveness in the sustained presence of ATP.
In such a scenario, ATP could not bind to the receptor under acidic conditions and, on the other hand, already bound ATP would be displaced once the receptor is exposed to an acidic environment (e.g. in the endolysosomes). However, this model cannot explain why P2X4 re‐sensitization is reduced in the presence of its ligand ATP and expression of H286A results in a reduced EC50 and Hill slope for ATP‐induced activation of P2X4 (Clarke et al. 2000; Tvrdonova et al. 2014). Inhibition of P2X4 re‐sensitization in the presence of ATP suggests the interaction of ATP with the channel. Whether this occurs via the ATP or an additional binding site is unclear. Also, it has been postulated that the H286 residue is located too far from the putative ATP binding site to affect ATP binding. It has been suggested that protonation affects intramolecular signal transmission from the ATP pocket to the gate and hence gating rather than ATP binding (Coddou et al. 2011; Tvrdonova et al. 2014). From our data, we can not determine a direct effect of protonation on gating. Interestingly, however, our histidine screen suggested stronger effects of H286 and H241. Both histidines are located adjacent to the ATP binding pocket within the fully assembled receptor and hence a yet unknown interaction between protonation and ATP binding can not be excluded. Future studies will be required to further mechanistically delineate the effects of acidic pH on unbinding of the ligand, gating, or the as yet unknown mechanisms required for receptor re‐sensitization. One possible approach could be to directly monitor ATP unbinding under control and acidic conditions (Bhargava et al. 2013).
Our observation that the presence of ATP under acidic conditions inhibits receptor re‐sensitization is also in contrast to a recent study reporting that an increase in the lysosomal pH results in activation of lysosomal P2X4 receptors by lysosomal ATP (Huang et al. 2014). However, in these experiments, a strong response was only observed when high concentrations of ATP (0.1–1 mm) were applied to the organelle lumen via the electrode, leaving the possibility that lysosomal P2X4 receptors were not directly exposed to (i.e. shielded from) endogenous ATP in these organelles, possibly as a result of compartmentalization. Alternatively, additional factors could impact on receptor re‐sensitization or ATP activity in an intact organelle under acidic conditions.
Irrespective of the molecular mechanisms of receptor re‐sensitization, our results may, at least in part, explain the observed difference in the peak current ‘ run‐down’ between whole‐cell and perforated patch recordings. This ‘run‐down’ has been attributed to a wash‐out of diffusible cytosolic factors regulating receptor re‐sensitization (Fountain & North, 2006; Zemkova et al. 2015). It is tempting to speculate and also quite conceivable that, under perforated conditions, soluble cytosolic regulatory proteins of vesicular trafficking (e.g. dynamin, GTP, small GTPases) are washed out. However, from our results, we cannot determine whether the diffusible cytosolic factors lost in the whole‐cell configuration are solely affecting the recycling‐dependent re‐senzitization of P2X4 receptors, or whether additional factors affecting gating and/or priming of the receptor directly are also required.
Our observation indicating that currents were often increased following the pH‐wash compared to the initial ATP evoked response is probably a result of surface expression of a fraction of non‐naïve receptors (receptors that have already been exposed to ATP before the start of the experiment) and have not yet been internalized/recycled. This is also supported by our observation that currents were often increased when cells expressing internalization‐deficient P2X4(Y378F)‐EGFP were primed and stimulated immediately after the initial ATP application at the start of the experiment. Similar but even stronger effects were observed in cells treated with bafilomycin A1 (Fig. 3 D). Again, this could be a result of premature exposure. However, the effect of bafilomycin A1 might also be stronger for the two reasons: (i) the Y378F mutant does not fully abrogate receptor internalization, and hence some residual (∼20–30%) recycling remains (Royle et al. 2002), resulting in partial re‐sensitization during the cell culture period, and (ii) it has been demonstrated that P2X4 can be activated by intravesicular ATP in acidic organelle upon neutralization of the intravesicular pH (Fois et al., 2018; Huang et al. 2014), and hence it is conceivable that P2X4 receptors are not only not re‐sensitized in bafilomycin treated cells, but also that intracellular P2X4 receptors are activated before insertion into the membrane.
Priming (potentiation) of P2X4 currents has also been reported following treatment with ivermectin (Toulme et al. 2006). Experimental data, as well as modelling approaches, attributed this effect of ivermectin in part to transferring channels to a sensitized state that also reduces internalization (Toulme et al. 2006; Zemkova et al. 2015). This is probbaly not the case for the priming effect by acidification. When we expressed internalization‐deficient P2X4(Y378F)‐GFP in TsA cells, priming could still be observed. This is in contrast to observations made following ivermectin treatment where no potentiation was observed for the same mutant of P2X4.
In sum, our data suggest a model where re‐cycling of P2X4 receptors via acidic organelles is essential for receptor re‐sensitization and receptor function. This depends on protonation and de‐protonation of critical histidine residues in the extracellular loop of P2X4 and requires a spatial or functional segregation from the P2X4 ligand ATP (Fig. 6).
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
MF designed the study. GF, KJF, CK, MiF and MF performed experiments and analysed data. PD, KJF and MF wrote the manuscript. All authors approved the final manuscript submitted for publication.
Funding
This work was supported by a grant from the Ministry of Science, Research and the Arts of Baden‐Württemberg (Az: 32‐7533.‐6‐10/15/5) and the Deutsche Forschungsgemeinschaft (DFG), grants SFB 1149/1, A05 to MF and DI 1402/3‐1 to PD.
Biographies
Giorgio Fois obtained his MSc in Biology at the University of Milan, Italy. He subsequently moved to University of Ulm with a Marie‐Curie‐Fellowship to study mechanically‐induced Ca2+‐signalling in lung cells. Under the supervision of Professor Dietl, he obtained his PhD in human biology. Subsequently, he has joined the group of Professor Frick to investigate the role of P2X4 channels and purinergic signalling in lung epithelial cells.

Karl Föhr is senior scientist at the department of Anesthesiology at the University Hospital of Ulm, Germany. During his PhD, he investigated intracellular Ca2+ regulation using ion selective electrodes and permeabilized cell preparations. Afterwards, he worked for 5 years in the Department of Biophysics at the NMI (Reutlingen). He returned to the University of Ulm for his habilitation at the Department of Physiology. In 1999, he switched to his current position with a research focus on drug‐receptor (ion channel) interactions.
These authors contributed equally to this work.
References
- Bhargava Y, Nicke A & Rettinger J (2013). Validation of Alexa‐647‐ATP as a powerful tool to study P2X receptor ligand binding and desensitization. Biochem Biophys Res Commun 438, 295–300. [DOI] [PubMed] [Google Scholar]
- Bobanovic LK, Royle SJ & Murrell‐Lagnado RD (2002). P2X receptor trafficking in neurons is subunit specific. J Neurosci 22, 4814–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Fredholm BB, North RA & Verkhratsky A (2010). The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 199, 93–147. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zhong XZ, Zou Y, Murrell‐Lagnado R, Zhu MX & Dong XP (2015). Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J Cell Biol 209, 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CE, Benham CD, Bridges A, George AR & Meadows HJ (2000). Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol 523, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro‐Toro JP & Stojilkovic SS (2011). Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63, 641–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Binns DD, Ueda H, Schmid SL & Baba T (2001). Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell 12, 2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fois G, Hobi N, Felder E, Ziegler A, Miklavc P, Walther P, Radermacher P, Haller T & Dietl P (2015). A new role for an old drug: Ambroxol triggers lysosomal exocytosis via pH‐dependent Ca(2)(+) release from acidic Ca(2)(+) stores. Cell Calcium 58, 628–637. [DOI] [PubMed] [Google Scholar]
- Fois G, Winkelmann VE, Bareis L, Staudenmaier L, Hecht E, Ziller C, Ehinger K, Schymeinsky J, Kranz C & Frick M (2018). ATP is stored in lamellar bodies to activate vesicular P2X4 in an autocrine fashion upon exocytosis. J Gen Physiol 150, 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ & North RA (2006). A C‐terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem 281, 15044–15049. [DOI] [PubMed] [Google Scholar]
- Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CR & North RA (2007). An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature 448, 200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller T, Auktor K, Frick M, Mair N & Dietl P (1999). Threshold calcium levels for lamellar body exocytosis in type II pneumocytes. Am J Physiol 277, L893‐L900. [DOI] [PubMed] [Google Scholar]
- Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, Murrell‐Lagnado R & Dong XP (2014). P2X4 forms functional ATP‐activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem 289, 17658–17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J & Helenius A (2011). Endosome maturation. EMBO J 30, 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek‐Hajek K, Lorinczi E, Hausmann R & Nicke A (2012). Molecular and functional properties of P2X receptors – recent progress and persisting challenges. Purinergic Signal 8, 375–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS & North RA (2006). P2X receptors as cell‐surface ATP sensors in health and disease. Nature 442, 527–532. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG & Lippincott‐Schwartz J (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Jones S, Roberts JA, Mahaut‐Smith MP & Evans RJ (2012). Heat shock protein 90 inhibitors reduce trafficking of ATP‐gated P2X1 receptors and human platelet responsiveness. J Biol Chem 287, 32747–32754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linely JE (2013). Perforated whole‐cell patch‐clamp recording. Methods Mol Biol 998, 149–157. [DOI] [PubMed] [Google Scholar]
- Liu X, Ma W, Surprenant A & Jiang LH (2009). Identification of the amino acid residues in the extracellular domain of rat P2X(7) receptor involved in functional inhibition by acidic pH. Br J Pharmacol 156, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph AG, Udvardi PT, Schaz U, Henes C, Adolph O, Weigt HU, Fegert JM, Boeckers TM & Fohr KJ (2010). Atomoxetine acts as an NMDA receptor blocker in clinically relevant concentrations. Br J Pharmacol 160, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C & Kirchhausen T (2006). Dynasore, a cell‐permeable inhibitor of dynamin. Dev Cell 10, 839–850. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA & Rothman JE (1998). Visualizing secretion and synaptic transmission with pH‐sensitive green fluorescent proteins. Nature 394, 192–195. [DOI] [PubMed] [Google Scholar]
- Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, Timmler M & Frick M (2011a). Fusion‐activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc Natl Acad Sci U S A 108, 14503–14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, Timmler M & Frick M (2011b). Fusion‐activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklavc P, Thompson KE & Frick M (2013). A new role for P2X receptors as modulators of lung surfactant secretion. Front Cell Neurosci 7, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA (2012). Lysosomal acidification mechanisms. Annu Rev Physiol 74, 69–86. [DOI] [PubMed] [Google Scholar]
- North RA (2002). Molecular physiology of P2X receptors. Physiol Rev 82, 1013–1067. [DOI] [PubMed] [Google Scholar]
- Qureshi OS, Paramasivam A, Yu JC & Murrell‐Lagnado RD (2007). Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci 120, 3838–3849. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Vial C, Digby HR, Agboh KC, Wen H, Atterbury‐Thomas A & Evans RJ (2006). Molecular properties of P2X receptors. Pflugers Arch 452, 486–500. [DOI] [PubMed] [Google Scholar]
- Robinson LE & Murrell‐Lagnado RD (2013). The trafficking and targeting of P2X receptors. Front Cell Neurosci 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L, Stirling CJ & Woodman PG (1994). Multiple N‐ethylmaleimide‐sensitive components are required for endosomal vesicle fusion. Mol Biol Cell 5, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Bobanovic LK & Murrell‐Lagnado RD (2002). Identification of a non‐canonical tyrosine‐based endocytic motif in an ionotropic receptor. J Biol Chem 277, 35378–35385. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S & Meyer T (1998). Receptor‐induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 8, 343–346. [DOI] [PubMed] [Google Scholar]
- Stoop R, Surprenant A & North RA (1997). Different sensitivities to pH of ATP‐induced currents at four cloned P2X receptors. J Neurophysiol 78, 1837–1840. [DOI] [PubMed] [Google Scholar]
- Surprenant A & North RA (2009). Signaling at purinergic P2X receptors. Annu Rev Physiol 71, 333–359. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Korbmacher JP, Hecht E, Hobi N, Wittekindt OH, Dietl P, Kranz C & Frick M (2013). Fusion‐activated cation entry (FACE) via P2X(4) couples surfactant secretion and alveolar fluid transport. FASEB J 27, 1772–1783. [DOI] [PubMed] [Google Scholar]
- Toulme E, Soto F, Garret M & Boue‐Grabot E (2006). Functional properties of internalization‐deficient P2X4 receptors reveal a novel mechanism of ligand‐gated channel facilitation by ivermectin. Mol Pharmacol 69, 576–587. [DOI] [PubMed] [Google Scholar]
- Tvrdonova V, Rokic MB, Stojilkovic SS & Zemkova H (2014). Identification of functionally important residues of the rat P2X4 receptor by alanine scanning mutagenesis of the dorsal fin and left flipper domains. PLoS ONE 9, e112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF & Burnstock G (1999). Modulation of ATP‐responses at recombinant rP2X4 receptors by extracellular pH and zinc. Br J Pharmacol 126, 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chai H, Ehinger K, Egan TM, Srinivasan R, Frick M & Khakh BS (2014). Imaging P2X4 receptor subcellular distribution, trafficking, and regulation using P2X4‐pHluorin. J Gen Physiol 144, 81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ & Maxfield FR (1984). Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J Cell Biochem 26, 231–246. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M & Tashiro Y (1991). Bafilomycin A1, a specific inhibitor of vacuolar‐type H(+)‐ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266, 17707–17712. [PubMed] [Google Scholar]
- Zemkova H, Khadra A, Rokic MB, Tvrdonova V, Sherman A & Stojilkovic SS (2015). Allosteric regulation of the P2X4 receptor channel pore dilation. Pflugers Arch 467, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]


