Abstract
The data presented here pertain to a research article entitled “Structural characterization and dynamics of globotetraosylceramide in vascular endothelial cells under TNF-α stimulation” (Okuda et al., 2010). The present article provides additional structural and gene expression data for the characterization of a TNF-α–inducible glycosphingolipid, globotetraosylceramide (Gb4), in vascular endothelial cells. (i) Structural details of Gb4 in lipid raft–enriched cell membranes were determined by MALDI-TOF MS. These analyses identified Gb4 with very-long-chain fatty acids as the major molecular species in this fraction, and the expression levels of whole molecular species of Gb4 with different fatty acid structures in the membrane are uniformly upregulated by TNF-α stimulation. (ii) The expression levels of genes encoding enzymes for synthesis of the ceramide portion of Gb4 were analyzed by real-time PCR. These assays revealed that TNF-α stimulation promotes transcription of the Elovl1 and Cers5 genes, which are involving in the synthesis of Gb4 with very-long-chain fatty acids. Collectively, these results indicate that TNF-α regulates glycosphingolipid synthesis and lipid raft formation in vascular endothelial cells via transcriptional up-regulation of related genes. These data thus provide new insights useful for understanding the molecular basis of inflammation-associated pathology in vascular endothelia.
Specifications table
| Subject area | Cell biology |
| More specific subject area | Vascular endothelial cells, inflammation, glycosphingolipids, lipid rafts, fatty acid elongase, ceramide synthase |
| Type of data | Images and tables |
| How data was acquired | MALDI-TOF MS and real-time PCR |
| Data format | Raw and analyzed data |
| Experimental factors | Vascular endothelial cells stimulated by TNF-α. |
| Experimental features | Lipid raft–enriched cell membranes were prepared by sucrose density gradient centrifugation in the presence of Triton X-100; purified membrane-associated glycosphingolipids were analyzed by MALDI-TOF MS using α-CHCA as the matrix. |
| cDNA was prepared from total cellular RNA, and the expression level of target genes was analyzed by real-time PCR using fluorescent-labeled hydrolysis probes. | |
| Data source location | Bioproduction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Central 6, Tsukuba |
| Data accessibility | The data are available with this article. |
| Related research article | T. Okuda, et al. Structural characterization and dynamics of globotetraosylceramide in vascular endothelial cells under TNF-α stimulation. Glycoconj. J. 27 (2010)287–296.[1] |
Value of the data
-
•
The data provide new insights into the molecular dynamics of glycosphingolipids in lipid rafts under inflammatory conditions, which can be of value to researchers in related fields.
-
•
Transcriptional up-regulation of Elovl1 and Cers5 in response to TNF-α stimulation is a novel finding.
-
•
These data can be compared to other scientific data addressing the effects of TNF-α on various cells and tissues.
-
•
The data and protocols provided here support other researchers investigating vascular inflammation and glycosphingolipids.
1. Data
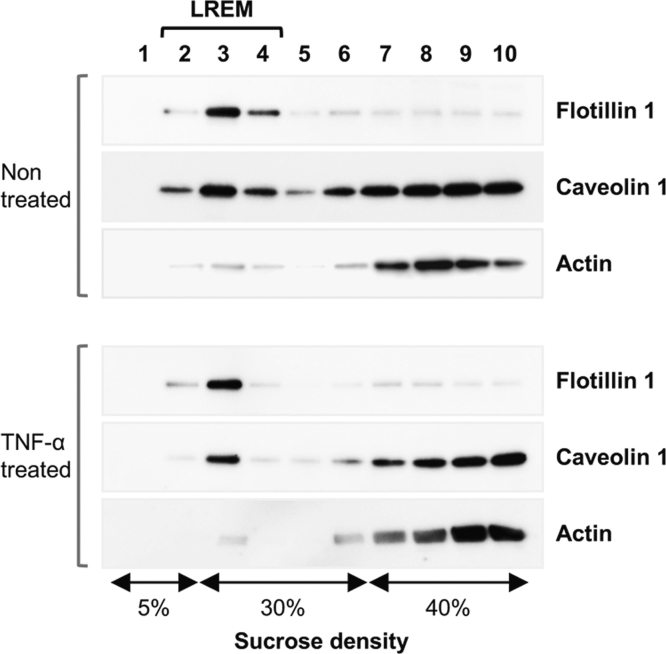
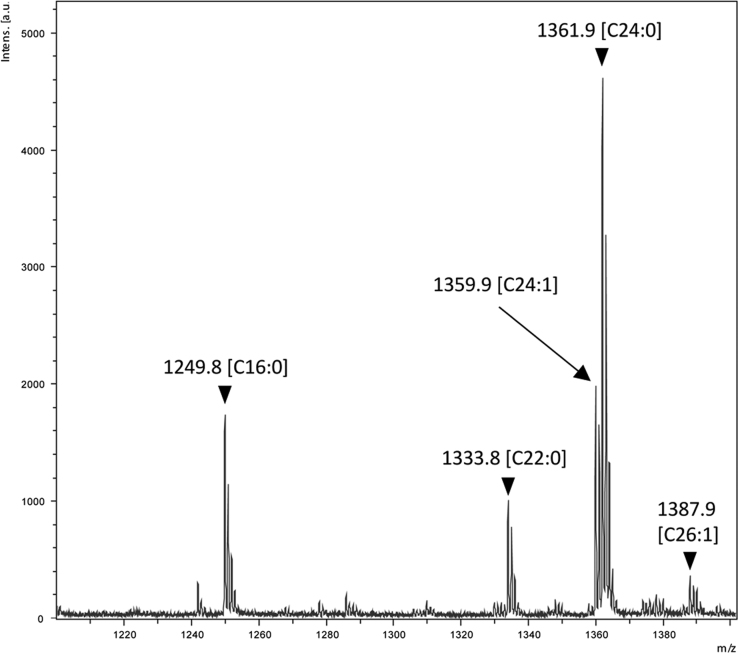
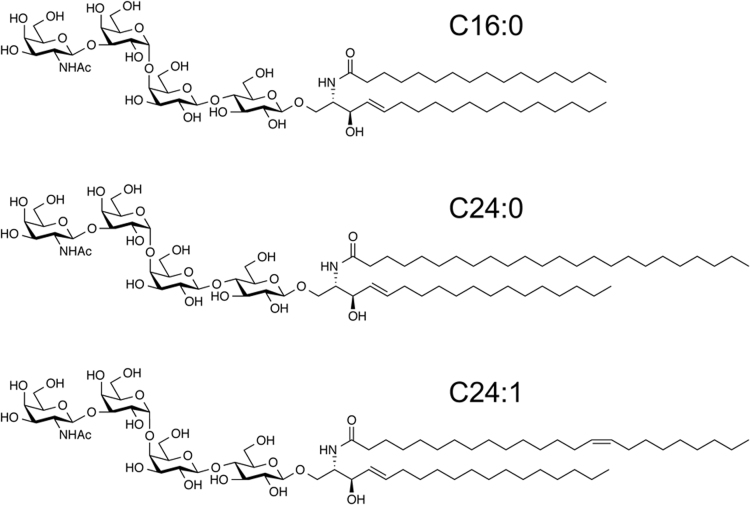
For MALDI-TOF MS analyses, lipid raft–enriched membranes (LREMs) of non-treated vascular endothelial cells (ECs) were prepared by sucrose density gradient centrifugation using 1% triton X-100, and fractions enriched in the lipid raft marker flotlline 1 were isolated as the LREM (Fig. 1). The LREM also partially included the caveolae marker caveolin 1. Glycosphingolipids were purified from the LREM fraction and the molecular composition of globotetraosylceramide (Gb4), a TNF-α–inducible glycosphingolipid in EC [1], was determined by MALDI-TOF MS. In positive-ion mass spectra, molecular ions corresponding to Gb4 were detected: [M+Na]+ (m/z 1249.8–1387.9). A representative mass spectrum of Gb4 in the LREM is shown in Fig. 2, and the results are summarized in Table 1. Major peaks were detected at m/z 1249.8, 1359.9, and 1361.9, corresponding to Gb4 with 4-sphingosine and C16:0 fatty acid, C24:1 fatty acid, and C24:0 fatty acid, respectively. These chemical structures are shown in Fig. 3. Minor peaks were detected at m/z 1333.8 and 1387.9, corresponding to Gb4 with 4-sphingosine and C22:0 fatty acid and C26:1 fatty acid, respectively. The MS data were statistically compared with data pertaining to TNF-α–treated ECs [1]. The intensity of these signals increased uniformly in TNF-α–treated ECs, and the total signal intensity increased significantly, by over 2-fold, compared to that of non-treated ECs. In contrast to the whole cell membrane [1], the ratio of these molecular species of Gb4 was unchanged, and the ratio in the LREM of ECs was close to that in the whole cell membrane of TNF-α–treated ECs [1].
Fig. 1.
Prepared lipid raft–enriched membrane fraction. Lipid raft markers, flotlline 1 and caveolin 1, were detected by immunoblotting, as described in the Section 2. Actin was used an internal control. LREM, lipid raft–enriched membrane.
Fig. 2.
Representative mass spectrum of Gb4 purified from a lipid raft–enriched membrane.
Table 1.
Mass spectra of Gb4 detected in LREMs.
| MS | Ceramide | Relative rate (%) |
Signal intensity (×10−3) |
||
|---|---|---|---|---|---|
| [M+Na]+ | Non | TNF | Non | TNF | |
| 1249.8 | C16:0 | 18.25 ± 4.68 | 13.31 ± 5.27 | 1.62 ± 0.13 | 2.72 ± 1.62 |
| 1333.8 | C22:0 | 10.46 ± 1.70 | 11.39 ± 0.89 | 0.98 ± 0.34 | 2.32 ± 0.78 |
| 1359.9 | C24:1 | 22.24 ± 3.31 | 26.51 ± 0.73 | 2.00 ± 0.17 | 5.33 ± 1.59 |
| 1361.9 | C24:0 | 45.36 ± 5.79 | 44.37 ± 5.58 | 4.23 ± 1.35 | 8.86 ± 2.58 |
| 1387.9 | C26:1 | 3.69 ± 0.40 | 3.42 ± 0.74 | 0.34 ± 0.10 | 0.68 ± 0.21 |
| Total | 9.17 ± 1.90 | 20.1 ± 5.88* | |||
Means ± S.D.; n = 3.
P < 0.05, non-treated cells vs TNF-α–treated cells. Non, non-treated cells; TNF, TNF-α–treated cells.
Fig. 3.
Chemical structures of Gb4 with C16:0 or C24 fatty acids.
For real-time PCR analyses, we examined six candidate genes (Elovl1, Elovl3, Elovl6, Cers2, Cers5, and Cers6) involved in the synthesis of Gb4 with C16 or C24 fatty acids. Elovl1 and Elovl3, which encode fatty acid elongase 1 and 3, respectively, mediate elongation of C18:0 and C20:1 acyl-CoAs to very-long-chain fatty acids, including C24:0 and C24:1 [2]. ELovl6 encodes fatty acid elongase 6, which mediates elongation of C16:0 acyl-CoA to C18:0 and C20:1 [2]. The Cers2 gene encodes ceramide synthase 2, which catalyzes the incorporation of C24:0 and C24:1 acyl-CoAs into sphingosine [3]. Cers5 and Cers6 encode ceramide synthase 5 and 6, respectively, which catalyze the incorporation of C16:0 acyl-CoA into sphingosine [3]. Ceramide synthase 5 can dimerize with ceramide synthase 2 to enhance the activity of ceramide synthase 2 [4]. The results of real-time PCR analyses are summarized in Table 2. Elovl1 transcripts were clearly detected in non-treated ECs, whereas Elovl3 transcripts were barely detectable, and Elovl1 expression was significantly up-regulated by TNF-α stimulation. TNF-α stimulation tended to increase the expression of Cers2, and significant up-regulation of Cers5 expression was observed following TNF-α stimulation. These results correlate with increased expression of Gb4 with C24 fatty acids in TNF-α–treated ECs. No significant changes were observed in the expression levels of other target genes, indicating that up-regulated expression of Gb4 with C24 fatty acids is regulated primarily by Elovl1 and CerS5.
Table 2.
Real-time PCR analysis of target gene expression.
| Relative expression |
TNF/Non | P-value | ||
|---|---|---|---|---|
| Non | TNF | |||
| Elovl1 | 3.41 ± 0.71 | 58.64 ± 17.39 | 17.20 | 0.008** |
| Elovl3 | n.d. | n.d. | ||
| Elovl6 | 2.66 ± 1.41 | 2.96 ± 2.07 | 1.11 | 0.820 |
| Cers2 | 0.17 ± 0.04 | 2.46 ± 1.55 | 14.75 | 0.059 |
| Cers5 | 0.64 ± 0.08 | 15.97 ± 5.48 | 24.98 | 0.011* |
| Cers6 | 2.87 ± 0.89 | 5.04 ± 1.83 | 1.75 | 0.095 |
The relative expression of target genes is shown as the ratio of expression relative to that of the internal control gene, St3gal5. As the level of St3gal5 expression in non-treated and TNF-α–treated ECs was more stable than that of general reference genes (Gapdh and Actb), it was used as the internal control in this experiment. Mean ± S.D.; n = 4 from two independent experiments.
P< 0.05,
P < 0.01, non-treated vs TNF-α–treated. Non, non-treated cells; TNF, TNF-α–treated cells; n.d., not detected.
2. Experimental design, materials, and methods
2.1. Cell culture
ECs (HUVEC; KURABO, Osaka, Japan) were maintained in HuMedia-EG2 (KURABO) culture medium, as reported previously [5]. The medium was replaced with fresh HuMedia-EG2 containing 20 ng/ml of TNF-α, and the cells were incubated for 12 or 24 h prior to MALDI-TOF MS or real-time PCR analysis, respectively.
2.2. Preparation of LREMs
The detergent-insoluble membrane fraction was isolated from ECs stimulated with TNF-α using sucrose density gradient centrifugation in the presence of Triton X-100 [1]. Protein markers for caveolae/lipid raft or actin protein in each fraction were detected by immunoblotting using mouse IgG1 monoclonal antibodies (BD Biosciences, Franklin Lakes, NJ), anti-caveolin 1 (mAb 2297), anti-flotilline 1 (mAb 18), and anti-Actin (mAb C4) as appropriate.
2.3. Glycosphingolipid extraction
Glycosphingolipid extraction from LREM was performed as reported previously [1]. In brief, total lipids from the LREM were sequentially extracted using the chloroform/methanol/water 2:1:0 and 1:2:0.8 (v/v/v), respectively, and glycosphingolipids were separated by column chromatography using Iatrobeads 6RS-8060 (Mitsubishi Kagaku Iatron, Tokyo, Japan).
2.4. MALDI-TOF MS
Mass spectrometry analysis of glycosphingolipids was carried out using MALDI-TOF MS according to a previously published method [1].
2.5. Real-time PCR
Real-time PCR analyses were performed as reported previously [6] with slight modifications which used fluorescent-labelled hydrolysis probes. Amplification and quantification of target gene cDNAs were performed using a Light Cycler® 480 II system with gene-specific primers, single hydrolysis probes (Universal ProbeLibrary Probes; Roche, Basel, Switzerland) and a reaction mixture containing Light Cycler® 480 Probe Master (Roche), according to the manufacturer׳s instructions. The gene-specific primers and single hydrolysis probes used in this study are summarized in Table 3.
Table 3.
Primers and probes used for real-time PCR analyses.
| Gene (numbera) | Primer sequence | Probe sequence (Probe numberb) |
|---|---|---|
| Elovl1 | Forward: 5′-ACTTCTCTCTGGCCCTGCT | GGATGGAG |
| (NM_022821) | Reverse: 5′-TCACCTCTTGGTACAAGTTCACA | (#58) |
| Elovl3 | Forward: 5′-AACCTGCAAGGGCCTCTC | CTTCTGCC |
| (NM_152310) | Reverse: 5′-TAATGCCCCACATCCTCACT | (#29) |
| Elovl6 | Forward: 5′-TTTGAACTGAGGAAGCCATTAGT | CTGGTCTC |
| (NM_024090) | Reverse: 5′-CAGTTCGAAGAGCACCGAAT | (#54) |
| Cers2 | Forward: 5′-AGACGGAGTACACGGAGCAG | GCTCCAGA |
| (NM_022075) | Reverse: 5′-CGTTCCCACCAGAAGTAATCA | (#50) |
| Cers5 | Forward: 5′-CACATCCTCTCGGTGTTCC | GGCGGCGG |
| (NM_147190) | Reverse: 5′-CAGGGTTTGGCAATAAATCG | (#70) |
| Cers6 | Forward: 5′-TCATGATTCAGCTGATGCTCTT | GGAGGCTG |
| (NM_203463) | Reverse: 5′-CACATTTTCTGAAACTTGGCATA | (#75) |
| St3gal5 | Forward: 5′-CTGCCTTTGACATCCTTCAGT | CTGGGGCC |
| (NM_003896) | Reverse: 5′-CGATTGTGGGGACGTTCTTA | (#57) |
Genbank accession number (http://www.ncbi.nlm.nih.gov/).
Probe number from the Universal Probe Library (Roche).
2.6. Statistical analysis
After determination of variance by the F-test, statistical significance was determined using the two-tailed Student׳s t-test, with statistical significance defined as follows: *P < 0.05, **P < 0.01.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science, Japan (JSPS KAKENHI Grant number 15H02907) and by the Cosmetology Research Foundation, Japan (Grant number 375).
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.09.059.
Transparency document. Supplementary material
Supplementary material.
.
References
- 1.Okuda T., Nakakita S., Nakayama K. Structural characterization and dynamics of globotetraosylceramide in vascular endothelial cells under TNF-α stimulation. Glycoconj. J. 2010;27:287–296. doi: 10.1007/s10719-009-9277-2. [DOI] [PubMed] [Google Scholar]
- 2.Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 2012;152:387–395. doi: 10.1093/jb/mvs105. [DOI] [PubMed] [Google Scholar]
- 3.Grösch S., Schiffmann S., Geisslinger G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012;51:50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Laviad E.L., Kelly S., Merrill A.H., Jr., Futerman A.H. Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 2012;287:21025–21033. doi: 10.1074/jbc.M112.363580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuda T., Furukawa K., Nakayama K. A novel, promoter-based, target-specific assay identifies 2-deoxy-d-glucose as an inhibitor of globotriaosylceramide biosynthesis. FEBS J. 2009;276:5191–5202. doi: 10.1111/j.1742-4658.2009.07215.x. [DOI] [PubMed] [Google Scholar]
- 6.Okuda T. PUGNAc treatment provokes globotetraosylceramide accumulation in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2017;487:76–82. doi: 10.1016/j.bbrc.2017.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.