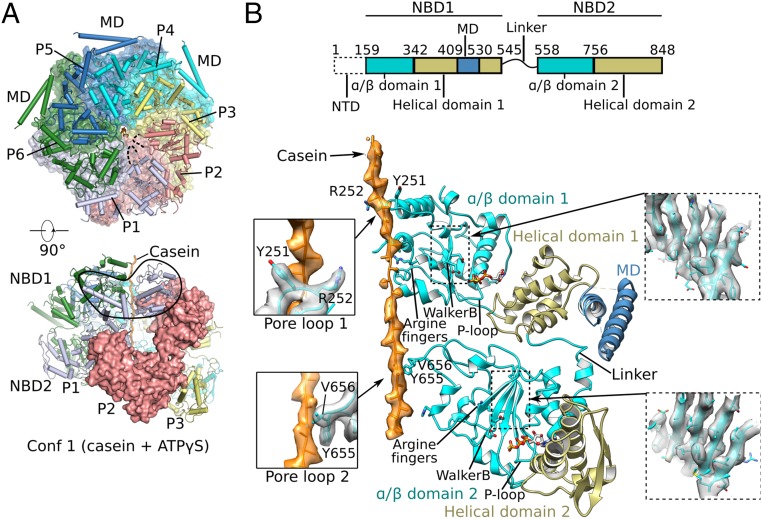
Fig. 2.
Cryo-EM structure of conformer 1 of Mtb ClpB complexed with ATPγS and casein. (A) Two orthogonal views of the atomic model of conformer 1 (corresponding to Fig. 1C). (Upper) The NBD2 ring is shown, overlapped with a semitransparent surface view. (Lower) Side view of conformer 1 with one protomer (P2) shown in surface view. (B, Upper) The domain architecture of ClpB. (Lower) Detailed structural elements of protomer P4 are shown in cartoon representation. The two α/β subdomains are shown in cyan, two helical subdomains in olive, MD domain in dark blue, and casein peptide in orange with electron density superimposed in semitransparent surface. Key features such as arginine fingers, Walker B motifs, P loops, and the linker between NBD1 and NBD2 are labeled. (Left Insets) Enlarged views of the interactions between the casein peptide and pore-loop 1 (Upper) and pore-loop 2 (Lower). (Right Insets) Electron density of the middle β-sheet of NBD1 (Upper) and NBD2 (Lower).