Summary
C5 plays a major role in complement activation; C5 convertase cleaves C5 into the pro‐inflammatory C5a, and C5b, the nidus for the formation of the lytic membrane attack complex. C5 is a major target for anti‐complement drugs, necessitating better methods for the study of C5 function. Purification of C5 is complicated; classical methods involve precipitation or pH shifts that result in functional loss and low yield. We here present a method for C5 purification using a novel anti‐C5 monoclonal antibody (mAb); RO7112689 (C5i mAb, SKY59), pH‐switch engineered to induce antibody–antigen dissociation in the acidic endosome (~ pH 5·5). RO7112689 was bound on an affinity column; applied serum was completely depleted of C5. Elution at pH 5 produced fully active C5 at 98% yield. The mAb also bound C5b in the C5b6 complex, preventing C5b6 binding to target membranes and enabling purification of C5b6 from activated serum. RO7112689 inhibited C5 in mouse serum and efficiently purified mouse C5. Used as capture, RO7112689 produced sensitive and specific assays for human and mouse C5. This novel antibody enables efficient production of intact, fully active, pure human and mouse C5, and quantification of C5 in these species. The demonstration that RO7112689 binds C5b6 adds an additional mechanism of membrane attack complex inhibition by this mAb.
Keywords: C5, complement, purification, RO7112689, SKY59
Abbreviations
- A
absorbance
- BSA
bovine serum albumin
- C5
complement component 5
- C5D
C5‐depleted serum
- CFD
complement fixation diluent
- ELISA
enzyme‐linked immunosorbent assay
- mAb
monoclonal antibody
- MAC
membrane attack complex
- NHS
normal human serum
- NMS
normal mouse serum
- NRS
normal rat serum
- NRbS
normal rabbit serum
- PBS
phosphate‐buffered saline
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
Introduction
The complement system, a network of over 30 fluid‐phase and cell‐membrane‐bound proteins and regulators, is a critical component of the innate immune defence against infection.1 Recent studies have broadened our view of complement – a mediator of pleiotropic effects including inflammation, modulation of adaptive immunity, metabolism and neuronal development.2, 3, 4 Complement activation pathways all lead to the formation of a C3‐cleaving enzyme (C3 convertase; C4b2a or C3bBb) that yields C3a and C3b; addition of C3b to the C3 convertase generates a C5 convertase (C4b2a3b; C3bBbC3b) that cleaves C5, releasing the pro‐inflammatory fragment C5a and initiating membrane attack complex (MAC) formation. C5 occupies a central position and, as a consequence, has been identified as an attractive target for anti‐complement drugs. Indeed, the anti‐C5 monoclonal antibody (mAb) eculizumab, a blocker of C5 cleavage by the convertase, is the most successful anti‐complement therapeutic to date and has transformed the outlook for patients with paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome. A recent review listed 34 anti‐complement drugs under development; of which over a third target C5.5
Characterization of current and new C5‐targeted drugs requires quality reagents, including C5‐depleted serum and pure C5; purification of C5 was described over 50 years ago, requiring multiple precipitation and chromatography steps, taking many days and generating poorly active protein in low yield.6 Later reports described more efficient methods, focused on reduction of time for purification; however, all involved precipitation and/or exposure to pH shifts, resulting in loss of C5 activity.7, 8, 9 A particular problem was the propensity of C5 to bind C6 when denatured by precipitation or pH shift during the early stages of purification, resulting in low yields of active protein.10, 11 Immunoaffinity methods using specific antibodies, commonplace for plasma proteins, are reported for C5;12 however, elution at extremes of pH significantly compromises C5 activity, in our hands resulting in > 50% activity loss despite rapid neutralization on elution.
We present an efficient, single‐step method of isolating fully functional C5 with a purification yield of 98%. A recently described humanized anti‐C5 mAb, RO7112689 (C5i mAb, SKY59), was selected by screening for antibodies showing strong binding to C5 at pH 7·4 but weak or negligible binding at pH 6.13 This pH‐dependent binding enables recycling of the antibody in vivo through dissociation from antigen at acidic pH in the endosome (~ pH 5·5). The antibody was also modified in the Fc region to eliminate the C1q‐binding site and hence its capacity to activate complement.13 C5 was completely depleted from serum with a single pass over Sepharose‐immobilized RO7112689 and efficiently eluted from the column at pH 5·5 with a yield of 98%. RO7112689 cross‐reacted with mouse C5 and efficiently purified C5 from mouse serum. As the capture mAb in enzyme‐linked immunosorbent assay (ELISA), RO7112689 allowed sensitive and specific quantification of human and mouse C5 in plasma.
RO7112689, like eculizumab, is reported to block C5 cleavage to prevent C5a and MAC generation;13, 14 we here show that RO7112689 also binds C5 in C5b6 and inhibits in a reactive lysis system, providing an additional mechanism of MAC inhibition that may enhance its therapeutic capacity, particularly in sites where C5 may be atypically activated such as the rheumatoid joint.
Materials and methods
Generation of RO7112689 affinity column
To generate an RO7112689 anti‐C5 affinity columns, RO7112689 antibody (50 mg; Roche, Basel, Switzerland) was diluted to 5 ml in coupling buffer (0·2 m Na2HCO3, 0·5 m NaCl pH 8·3), immediately injected into a pre‐activated 5‐ml HiTRAP N‐hydroxysuccinimide‐activated high‐performance column (GE Healthcare, Little Chalfont, UK) and incubated for 1 hr at room temperature. The column was blocked with 0·5 m ethanolamine pH 8·3 and washed into running buffer [Complement Fixation Diluent (CFD); Oxoid, ThermoFisher, Basingstoke, UK] containing 0·5 m NaCl; column eluate was retained to measure unbound antibody [bicinchoninic acid (BCA) assay, ThermoFisher]; coupling efficiency was > 90%.
Affinity purification of C5
Normal human serum (NHS) was prepared in‐house from healthy donors. Pooled NHS was applied undiluted to the RO7112689 column at a rate of 1 ml/min and 4‐ml fractions were collected for assessment of C5 depletion. The column was washed with four volumes of running buffer (CFD containing 0·5 m NaCl) then eluted using acetate buffer pH 5·5 containing 0·15 m NaCl. Protein‐containing fractions were collected, pooled and dialysed overnight at 4°C into CFD containing 0·5 m NaCl. Dialysed protein was divided into aliquot and stored at −80°. Seven separate purifications were performed over 14 months on the column (Table 1). Between uses the column was stored in phosphate buffered saline (PBS) containing 0·01% NaN3 at 4°.
Table 1.
Purifications performed to date for human and mouse C9 on the RO7112689 columns
| Purification no. | Column | Serum applied (ml) | Month/year | Calculated yield |
|---|---|---|---|---|
| Human | ||||
| 1 | 5 ml | 20 | 04/2017 | – |
| 2 | 5 ml | 30 | 05/2017 | 96% |
| 3 | 5 ml | 70 | 05/2017 | 97% |
| 4 | 5 ml | 180 | 05/2017 | 97% |
| 5 | 5 ml | 50 | 09/2017 | 98% |
| 6 | 5 ml | 350 | 11/2017 | 98% |
| 7 | 5 ml | 332 | 05/2018 | 98% |
| Mouse | ||||
| 1 | 1 ml | 2 ml | 02/2018 | 92% |
| 2 | 1 ml | 2 ml | 03/2018 | 93% |
The same 5‐ml column was used for all seven human C5 purifications. Yield was calculated as described in the Materials and methods for each purification (except for the first purification)
Normal mouse serum (NMS) was prepared from male mouse blood freshly harvested by cardiac puncture, collected into glass tubes, incubated at room temperature for 5 min then on ice for 20 min. Serum was collected, filtered and pooled. Mouse serum (2 ml) was applied to a 1‐ml HiTRAP column with 10 mg RO7112689 immobilized, made as above. The column was washed and eluted as above; eluted protein was dialysed and stored.
Characterization of purified proteins
C5 (human or mouse; 2 μg) was resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing and non‐reducing conditions on 7·5% PAGE gels, alongside commercially sourced human C5 (Complement Technology Inc, Tyler, TX). Gels were stained with Coomassie Blue dye (ThermoFisher). For Western blotting, 1 μg of C5 was resolved by SDS–PAGE under reducing and non‐reducing conditions as above, then electrophoretically transferred onto 0·45‐μm nitrocellulose membrane (GE Healthcare). After transfer, non‐specific sites on the membrane were blocked with 3% bovine serum albumin (BSA) in PBS containing 0·1% Tween‐20 (PBS‐T). After washing in PBS‐T, the membrane was incubated with the primary antibody; RO7112689 (at 1 μg/ml in 3% BSA PBS‐T) or polyclonal (goat) anti‐human C5 (CompTech, Texas, USA; A220; 2 μg/ml in 3% BSA PBS‐T). After washing, bound antibodies were detected by incubation with donkey anti‐human IgG‐horseradish peroxidase or rabbit anti‐goat IgG‐horseradish peroxidase conjugate (Jackson ImmunoResearch, W Baltimore Pike, West Grove, USA; 709‐036‐149, 305‐035‐045) at 1 : 5000 in PBS‐T. After washing, the blot was developed with enhanced chemiluminescence (GE Healthcare) and visualized by autoradiography.
Haemolytic assays
To determine the residual lytic capacity of C5‐depleted serum from the RO7112689 affinity column, antibody‐sensitized sheep erythrocytes (sheep blood from TCS Bioscience, Botolph Claydon, UK) were suspended in CFD at 2% and aliquots were placed in a 96‐well round‐bottomed plate (50 μl/well) followed by 50 μl/well of each C5‐depleted fraction or 10% NHS as positive control. Plates were incubated at 37° for 30 min, centrifuged and haemoglobin in the supernatant was measured by absorbance (A) at 405 nm. Percentage lysis was calculated according to: % Lysis = (Asample – Abackground)/(Amax – Abackground)*100%. To test the function of purified C5, protein was added back to C5‐depleted serum at various doses up to 75 μg/ml in undiluted C5‐depleted serum, then tested in haemolysis as above. For measurement of mouse serum haemolytic activity, sheep erythrocytes were additionally sensitized with mouse anti‐rabbit IgG (Invitrogen, Carlsbad, CA; #3123) to generate efficient activation of mouse complement. Assays were then performed as for human serum.
The inhibitory activity of RO7112689 in different species sera was investigated by haemolysis assay as described above. A serial dilution series of the antibody (100–0 μg/ml; 50 μl/well) was prepared in CFD, then sheep erythrocytes and serum (50 μl/well of each) were added as above. Serum dilution for each species was selected in preliminary experiments to give near‐complete haemolysis in the assay: NHS, 1·25%; male NMS, 20% (using the double‐sensitized cells as above); normal rat serum (NRS), 1·25%; normal rabbit serum (NRbS), 25%. The volumes of reagents, incubation times and assay conditions were as described above.
To test C5b6‐mediated reactive lysis, guinea‐pig erythrocytes at 2% in CFD (50 μl/well) were incubated (15 min, 37°) with 50 μl/well C5b6 (in‐house, 0·125 μg/ml in CFD) in a 96‐well round‐bottom plate. C7 (in‐house, 0·7 μg/well) was added and incubated for 15 min at 37°, then C8 and C9 (in‐house,1 μg/well of each) were added and incubated for 30 min at 37°. Supernatant was harvested and % Lysis was calculated as above. In some experiments, RO7112689 at various doses was pre‐incubated with C5b6 before exposure to guinea‐pig erythrocytes; in other experiments, RO7112689 at various doses was incubated with C5b6‐pre‐coated guinea‐pig erythrocytes before the addition of C7. Complement proteins C5, C6, C7, C8 and C9 were prepared in‐house by affinity purification from serum using specific monoclonal antibodies immobilized on Sepharose; C5b6 was prepared by incubating C5 and C6 with a fluid‐phase convertase comprising cobra venom factor and activated factor B.
ELISA for mouse and human C5
To develop an ELISA using RO7112689 as capture, Maxisorp plates (Nunc, Loughborough, UK) were coated with RO7112689 (1 μg/ml in bicarbonate buffer, pH 9·6) at 4° overnight; wells were blocked (1 hr at 37° with 2% BSA in PBS), washed in PBS‐T. Standard curves of purified proteins (human C5, C5b6 and C6, and mouse C5; stock concentrations established using the BCA assay), and mouse or human serum samples diluted in 0·2% BSA PBS, were added in duplicate and incubated for 1 hr at 37°. Wells were washed with PBS‐T then incubated (1 hr, 37°) with polyclonal (goat) anti‐human C5 cross‐reactive with mouse C5 (CompTech; 2 μg/ml in PBS‐T). After washing, wells were incubated with secondary antibody (rabbit anti‐goat‐horseradish peroxidase; Jackson ImmunoResearch) for 1 hr at 37°. After washing, plates were developed using o‐phenylenediamine dihydrochloride (SIGMAFAST™; Sigma‐Aldrich, St Louis, MO) and absorbance (492 nm) was measured. Protein concentrations (μg/ml) in serum samples were automatically calculated by reference to the standard curve using graphpad prism (GraphPad, La Jolla, CA, USA). Assay detection limits, working ranges and assay performance were determined as described.15
Results
C5 is purified to homogeneity in a single step on the RO7112689 column
RO7112689‐purified C5 demonstrated similar banding under reducing and non‐reducing conditions when compared with commercially available C5 (CompTech), a single band in non‐reduced SDS–PAGE of 190 000 MW and two bands under reducing conditions; 115 000 MW (C5α chain) and 75 000 MW (C5β chain) (Fig. 1a,b). No other bands were detected in the C5 preparation, confirming the high purity of the protein. Western blotting using RO7112689 confirmed that RO7112689 bound the C5β chain as reported previously13 (Fig. 1c). The average yield from seven separate purifications across 14 months on the same column (Table 1), based upon ELISA measurement of C5 concentration in pooled NHS (see below; 81·3 μg/ml), and concentration of final C5 pool (by BCA) was ~ 98% (e.g. for purification 6 : 350 ml serum, calculated available 28·45 mg; purified 27·7 mg).
Figure 1.
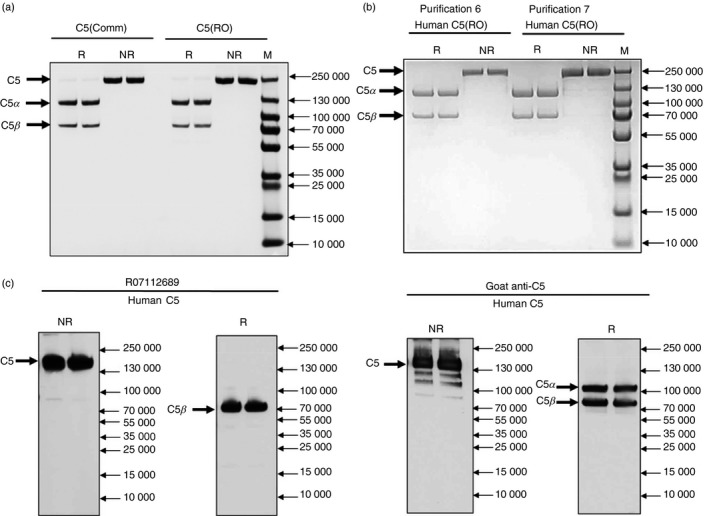
Characterization of purified C5. (a) SDS–PAGE of purified human C5 (2 μg) from purification #6 (Table 1) compared with commercial C5 (CompTech) on a 7·5% acrylamide gel; non‐reduced (NR), reduced with 5% β‐mercaptoethanol (R), proteins were stained with Coomassie Blue. NR; 190 000 MW (intact C5), R; 115 000 MW (C5α chain) and 75 000 MW (C5β chain). RO; RO7112689. (b) Human C5 (2 μg) from purifications #6 and #7 performed 6 months apart (Table 1) run as above to demonstrate the stability and reproducibility of the procedure. (c) Western blot of human C5. C5 (1 μg) was resolved on 7·5% PAGE under NR and R conditions and processed for Western blotting with RO7112689 or goat anti‐human C5 (CompTech). C5; NR; 190 000 MW (intact C5), R; 75 000 MW (C5β chain). Goat anti‐human C5 detected the native C5 in NR conditions and both C5α (115 000 MW) and C5β chains in R conditions, whereas RO7112689 detected C5 in NR conditions and only the C5β chain in R conditions.
Haemolytic assays demonstrated that lytic activity was completely removed from 350 ml of pooled NHS in a single pass over the column (Fig. 2a). Adding C5 to C5‐depleted serum (C5D) at physiological levels fully restored serum lytic activity, confirming that the C5 depletion was specific (Fig. 2b). C5D reconstituted with RO7112689‐purified C5 had a calculated the 50% haemolytic complement (CH50) of 63·4 Units, whereas C5D reconstituted with commercial C5 had a CH50 of 40·2 Units. Titration of C5 into a constant dilution of C5D confirmed that commercially sourced C5 was significantly less efficient at restoring haemolytic activity; the dose of C5 restoring to 50% haemolysis in 10% C5D was 221·5 ng/ml for RO7112689‐purified C5 and 714·7 ng/ml for commercial C5 (Fig. 2c). As little as 2 ng/ml RO7112689‐purified C5 conferred detectable haemolytic activity to 10% C5D (Fig. 2d).
Figure 2.
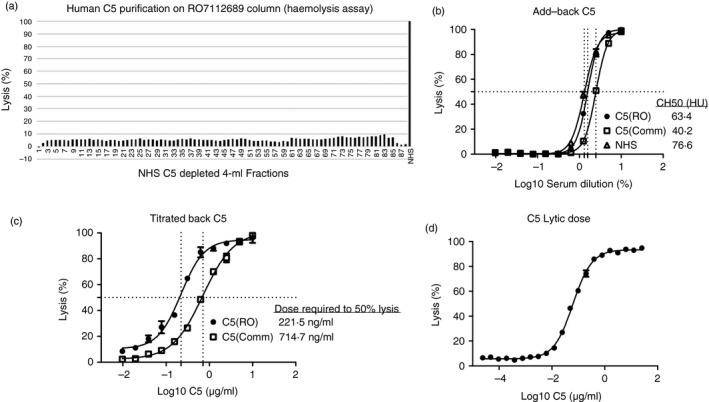
Haemolysis assays. (a) Haemolysis assay on run‐through fractions (each 4 ml) collected from pooled normal human serum (NHS) passed over the RO7112689 column; less than 10% lysis seen on each fraction; complete depletion of human C5. NHS (1 : 10) was used as the positive control (100% lysis). (b) Repletion with C5; C5 sourced either from RO7112689 purification or a commercial source was added back to neat C5‐depleted serum (C5D) at 75 μg/ml; both C5 sources fully restored haemolytic activity but commercial C5 restored serum was less lytic; calculated CH50 values were: RO7112689 C5, 63·4 Units; commercial C5, 40·2 Units. (c) SKY59‐purified C5 or commercial C5 were titrated back into 10% C5D to measure the dose that restored haemolysis to 50%. The 50% lytic dose of RO7112689‐purified C5 was 221·5 ng/ml, whereas that for commercial C5 was 714·7 ng/ml. (d) Titration to identify the lowest lytic dose of SKY59 purified C5 when added back to C5D; C5 at 2 ng/ml conferred measurable lytic capacity in 10% C5D. RO; RO7112689.
RO7112689 inhibits haemolysis in classical pathway and reactive lysis systems
Lytic inhibitory properties of RO7112689 were investigated using sheep erythrocytes and NHS at a dose selected to cause near‐100% target lysis. The dose of RO7112689 was titrated in the assay; the mAb efficiently inhibited lysis by NHS (Fig. 3a). At the selected dilution (1 : 80; 50 μl), each well contained ~ 50 ng C5 and near‐complete inhibition was obtained with ~ 25 ng mAb in the assay. In the reactive lysis system, pre‐incubation of C5b6 with RO7112689 efficiently blocked lysis on subsequent addition of C7‐C9 (complete inhibition at a molar ratio C5b6: RO7112689 of 1 : 2; Fig. 3e). In contrast, incubation of guinea‐pig erythrocytes pre‐coated with C5b6 with RO7112689 caused no inhibition of lysis on the addition of C7‐C9, even at the highest antibody doses (Fig. 3f).
Figure 3.
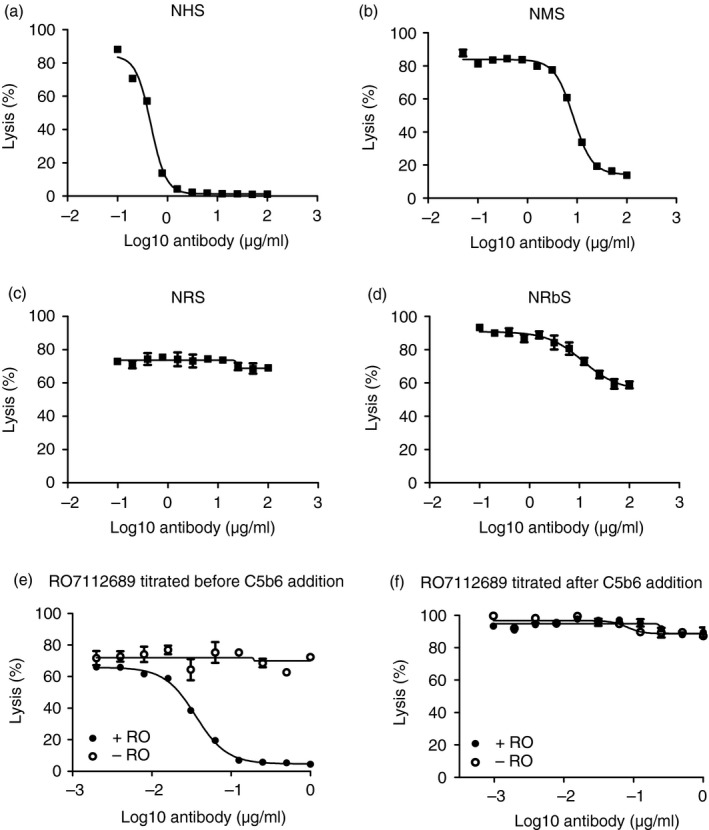
Cross‐species activity of RO7112689. RO7112689 inhibitory activity was tested in different species sera; human (a), mouse (male) (b), rat (c) and rabbit (d); serum doses were selected to give near‐maximum lysis in the absence of inhibitor (2, 20 1·25 and 25% respectively for these four species). RO7112689 was titrated from 100–0 μg/mL; strong inhibition of lysis was observed with human and mouse serum, weak inhibition with rabbit and no inhibition with rat. NHS, normal human serum; NMS, normal mouse serum; NRS, normal rat serum; NRbS, normal rabbit serum. Reactive lysis assays used guinea‐pig erythrocytes (GpE) and purified complement proteins; C5b6, C7, C8 and C9. (e) RO7112689 when added to GpE before C5b6 addition efficiently inhibited reactive lysis. (f) RO7112689 added after GpE were pre‐coated with C5b6 complex caused no inhibition of reactive lysis. RO7112689 addition, + RO; no antibody added, − RO.
RO7112689 inhibits mouse C5 and efficiently purifies C5 from mouse serum
Inhibition of haemolytic activity in NMS (male), NRS and NRbS by RO7112689 was tested as above. The mAb efficiently inhibited haemolysis by NMS; at the selected dilution (1 : 5; 50 μl), each well contained ~ 1000 ng C5 and near‐complete inhibition was obtained with ~ 500 ng mAb in the assay (Fig. 3b). Weak inhibition of NRbS and no inhibition of NRS was seen (Fig. 3c,d).
Passage of mouse serum over the RO7112689 column efficiently depleted C5 from the serum; elution of the column using conditions as described above for human‐produced pure mouse C5 in a single step (Fig. 4a). Western blotting of the purified mouse C5 confirmed RO7112689 binding to the β‐chain of mouse C5 (Fig. 4b).
Figure 4.
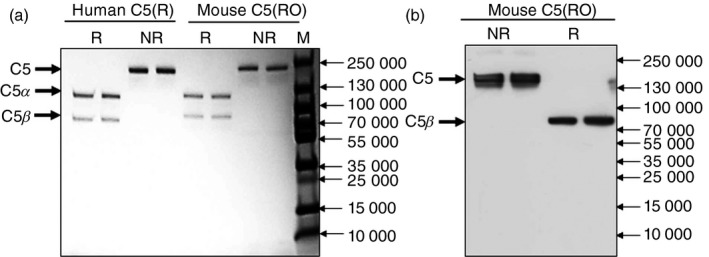
Purification of mouse C5 on RO7112689. (a) SDS–PAGE of purified mouse C5 (2 μg) on a 4–20% acrylamide gel; non‐reduced (NR), or reduced with 5% β‐mercaptoethanol (R), proteins were stained with Coomassie Blue. NR; 190 000 MW (intact C5), R; 115 000 MW (C5α chain) and 75 000 MW (C5β chain). (b) Western blot of mouse C5. C5 (1 μg) was resolved on 7·5% PAGE under NR and R conditions and processed for Western blotting with RO7112689. C5: NR; 190 000 MW (intact C5); R, 75 000 MW (C5β chain).
ELISA using RO7112689 enables quantification of C5 in NHS and NMS
Using RO7112689 as capture antibody and a mouse C5‐cross‐reactive polyclonal goat anti‐human C5 (CompTech) as detector generated a highly sensitive ELISA capable of measuring human and mouse C5 (Fig. 5a,b). The assay had a working range of 0·2–125 ng/ml and a detection limit of 0·24 ng/ml. The assay did not detect C3, C3a or C5a, tested using purified proteins (negative data not shown). NHS samples from 25 healthy donors and NMS samples from 11 C57BL/6 mice were measured. C5 in NHS was 79·6 μg/ml (range; 56·0–130·7), and in male NMS was 102·7 μg/ml (range; 92·7–130·6) (Fig. 5c). The assay detected C5b6 but not C6 (Fig. 5d); the signal for C5b6 was lower by ~ 50% compared with the same molar dose of C5, suggesting some hindrance of binding of the antibodies to C5b in the C5b6 complex. Binding of C5b6 in the ELISA supports the data, demonstrating capacity of RO7112689 to inhibit reactive lysis when pre‐incubated with C5b6.
Figure 5.
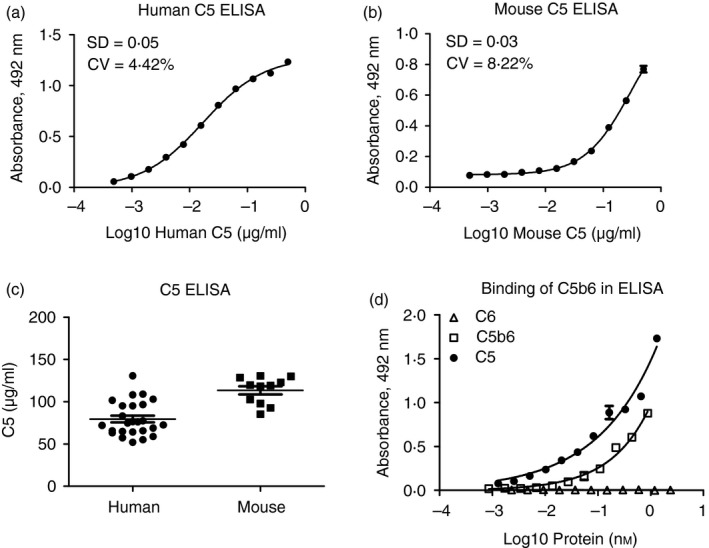
ELISA for human and mouse C5. A C5 sandwich ELISA was generated using RO7112689 as capture antibody and polyclonal anti‐human C5 as detection. Standard curves were generated using either human C5 (a) or mouse C5 (b). Normal human serum (NHS) samples from 25 healthy donors and normal mouse serum (NMS) samples from 11 male C57BL/6 mice were measured (c). The average level of C5 in NHS was 79·6 μg/ml (range; 56·0–130·7), and in NMS was 102·7 μg/ml (range; 92·7–130·6). The assay detected human C5 and C5b6, but not C6; signal intensities of C5b6 were ~ 50% less intense compared with the same amount of C5 (d).
Discussion
The explosion of interest in complement component C5 as a target for therapeutic intervention in complement‐mediated diseases has revealed a need to better characterize C5 activity and its interactions with current and future therapeutic agents.5 Such studies require pure, functionally intact C5 and C5‐depleted sera and would also be facilitated by the availability of rodent C5 protein and reagents. Purification of C5 has been challenging; early methods were laborious and inefficient with a very low recovery of active C5.6, 7, 8, 9 Current affinity purification methods are faster but are complicated by low yield (> 50%) and poor functional activity of the protein, a consequence of its pH lability and tendency to associate with C6 if denatured.10, 11, 12 Here we took advantage of a recently described anti‐C5 therapeutic mAb, RO7112689, that was selected to recycle in vivo by shedding its cargo when exposed to acidic pH (~ pH5·5) in the endosome.13 Such antibodies have been engineered to reduce therapeutic dose;16, 17 pH‐dependence to release soluble antigen in the endosome is an essential property.18 Importantly, the mAb had also been engineered in the Fc region to ablate C1q binding and hence complement‐activating capacity.13, 19 RO7112689 immobilized on an affinity column completely depleted C5 from a large pool of serum and the bound protein was eluted at pH 5·5 intact, pure, fully functional and at 98% yield. The successful isolation of a labile protein by using a pH‐switch mAb to avoid exposure of the protein to extremes of pH during affinity purification makes the case for similar strategies, using similar pH‐switch‐selected antibodies, for purification of other pH‐sensitive proteins. In the original description of RO7112689, it was stated that the mAb poorly cross‐reacted with mouse C5; however, no data were included.13 We have not formally measured the affinity of RO7112689 for mouse C5 but show clearly that it binds the mouse C5β chain and binds sufficiently strongly to block C5 activity in mouse serum and to enable single‐step purification of mouse C5 from serum. The cross‐reactivity also enabled the development of a capture ELISA that allowed quantification of C5 in mouse serum (mean of 102·7 μg/ml from 11 male mice), a useful tool for future studies.
The mechanism of action of RO7112689 was stated to be blockade of C5 cleavage by the C5 convertase; this mechanism is the same as that described for the first anti‐C5 therapeutic, eculizumab, despite the fact that these mAb bind different chains of C5.13, 14, 20 We found that RO7112689 also inhibited in a reactive lysis system using purified C5b6; when added to C5b6 before incubation with the target guinea‐pig erythrocytes, RO7112689 efficiently inhibited reactive lysis, suggesting that the mAb binds C5b6 and prevents its association with the membrane. This interpretation was supported by the finding that the mAb did not inhibit reactive lysis when added after binding of C5b6 to the target guinea‐pig erythrocytes, and by the demonstration that RO7112689 bound C5b6 in an ELISA. The capacity to inhibit C5b6‐mediated lysis might be an important second mode of action for this mAb, particularly given the growing list of situations where C5 is atypically activated by proteases to generate a C5b‐like fragment capable of triggering MAC assembly.21, 22, 23 Blockers of convertase‐mediated C5 cleavage may fail to block these pathologically important cleavages while C5b6 blockers may still inhibit MAC formation.
Disclosures
BPM and CLH have provided advice on complement to Roche. BPM is a consultant to GlaxoSmithKline, all fees are paid to Cardiff University. CLH is a past‐employee of GlaxoSmithKline and has various company consultancies; all company income is donated to the University for research and is not taken personally. Other authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Author contributions
WMZ performed all laboratory analyses, DW wrote the first draft of the manuscript, MS contributed expertise on C5 ELISA and analysis of haemolysis assays, CLH provided critical expertise on C5 purification, BPM conceived and planned the study and oversaw the data handling and manuscript preparation. All authors contributed to and have approved the final manuscript.
Acknowledgements
The author(s) declared receipt of the following financial support for the research, authorship and/or publication of this article: WMZ was supported by a PhD studentship from the Life Sciences Research Network Wales. The RO7112689 antibody was kindly provided by Roche (Basel, Switzerland).
References
- 1. Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov 2015; 14:857–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phieler J, Garcia‐Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol 2013; 25:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 2012; 35:369–89. [DOI] [PubMed] [Google Scholar]
- 4. Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol 2017; 188:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris CL. Expanding horizons in complement drug discovery: challenges and emerging strategies. Semin Immunopathol 2018; 40:125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nilsson UR, Mueller‐Eberhard HJ. Isolation of β IF‐Globulin from human serum and its characterization as the fifth component of complement. J Exp Med 1965; 122:277–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tack BF, Morris SC, Prahl JW. Fifth component of human complement: purification from plasma and polypeptide chain structure. Biochemistry 1979; 18:1490–7. [DOI] [PubMed] [Google Scholar]
- 8. Al Salihi A, Ripoche J, Pruvost L, Fontaine M. Purification of complement components by hydrophobic affinity chromatography on phenyl‐Sepharose. FEBS Lett 1982; 150:238–42. [Google Scholar]
- 9. DiScipio RG, Sweeney SP. The fractionation of human plasma proteins. II. The purification of human complement proteins C3, C3u, and C5 by application of affinity chromatography. Protein Expr Purif 1994; 5:170–7. [DOI] [PubMed] [Google Scholar]
- 10. Dessauer A, Rother U. The fifth component of complement (C5): purification without activation. Immunobiology 1983; 164:370–9. [DOI] [PubMed] [Google Scholar]
- 11. Chakravartis DN, Muller‐Eberhardb HJ. Biochemical characterization of the human complement protein C6 association with a‐thrombin‐like enzyme and absence of serine protease activity in cytolytically active C6. J Biol Chem 1988; 263:18306–12. [PubMed] [Google Scholar]
- 12. Giles JL, Choy E, van den Berg C, Morgan BP, Harris CL. Functional analysis of a complement polymorphism (rs17611) associated with rheumatoid arthritis. J Immunol 2015; 194:3029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukuzawa T, Sampei Z, Haraya K, Ruike Y, Shida‐Kawazoe M, Shimizu Y et al Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement‐mediated diseases. Sci Rep 2017; 7:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schatz‐Jakobsen JA, Zhang Y, Johnson K, Neill A, Sheridan D, Andersen GR. Structural basis for eculizumab‐mediated inhibition of the complement terminal pathway. J Immunol 2016; 197:337–44. [DOI] [PubMed] [Google Scholar]
- 15. Ingram G, Hakobyan S, Hirst CL, Harris CL, Loveless S, Mitchell JP et al Systemic complement profiling in multiple sclerosis as a biomarker of disease state. Mult Scler 2012; 18:1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S et al Antibody recycling by engineered pH‐dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 2010; 28:1203–7. [DOI] [PubMed] [Google Scholar]
- 17. Igawa T, Mimoto F, Hattori K. pH‐dependent antigen‐binding antibodies as a novel therapeutic modality. Biochim Biophys Acta 2014; 1844:1943–50. [DOI] [PubMed] [Google Scholar]
- 18. Igawa T, Haraya K, Hattori K. Sweeping antibody as a novel therapeutic antibody modality capable of eliminating soluble antigens from circulation. Immunol Rev 2016; 270:132–51. [DOI] [PubMed] [Google Scholar]
- 19. Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER et al An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 2014; 65:114–26. [DOI] [PubMed] [Google Scholar]
- 20. Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007; 25:1256–64. [DOI] [PubMed] [Google Scholar]
- 21. Foley JH, Walton BL, Aleman MM, O'Byrne AM, Lei V, Harrasser M et al Complement activation in arterial and venous thrombosis is mediated by plasmin. EBioMedicine 2016; 5:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber‐Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR et al Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med 2006; 12:682–7. [DOI] [PubMed] [Google Scholar]
- 23. Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B et al Molecular intercommunication between the complement and coagulation systems. J Immunol 2010; 185:5628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


